1. Background
- Berwick Bank Wind Farm Limited (BBWFL) is a wholly owned subsidiary of SSE Renewables Limited and will hereafter be referred to as ‘the Applicant’. The Applicant is developing the Berwick Bank Wind Farm (hereafter referred to as ‘the Project’) located in the outer Forth and Tay region ( Figure 1.1 Open ▸ ).
- The Project is located adjacent to the consented Forth and Tay offshore wind farms consisting of Seagreen 1 and Seagreen 1A Project to the north, Inch Cape to the northwest and Neart na Gaoithe to the west. ( Figure 1.1 Open ▸ ).
- The marine components of the Project, which are the focus of this technical report, include the Proposed Development array area and the Proposed Development export cable corridor ( Figure 1.1 Open ▸ ), hereafter referred to collectively as the ‘Proposed Development’.
- The Project will, if consented, provide an estimated 4.1 GW of renewable energy, making it one of the largest offshore wind farms in the world. Given the anticipated operational life span of 35 years, the development will make a critical contribution to Scotland’s renewable energy target of 11 GW of new offshore wind by 2030. Initially, pre-July 2021, the area was named as two separate sites, Marr Bank and Berwick Bank, but these have now been merged to a single site, Berwick Bank Wind Farm.
- Wind turbine capacity is yet to be confirmed but is expected to be between 14 – 24 MW. 1. The Proposed Development will comprise up to 307 wind turbines, with the final number of wind turbines dependent on the capacity of individual wind turbines used, and also environmental and engineering survey results. The PDE considers a range of wind turbines with parameters reflective of potential generating capacities, allowing for a degree of flexibility to account for any anticipated developments in wind turbine technology while still allowing the production of the MDS for the assessment of effects. Importantly, the minimum lower blade tip height is 37 m (above LAT) for all wind turbine options as an engineering design measure to reduce collision risk to seabirds.
- The site boundary of the Proposed Development balances maximising the potential for renewable energy generation whilst reducing environmental impacts.
- The purpose of this technical report is to put the potential impacts from the Project on seabirds in the context of the wider ecosystem. This is in response to the earlier Scoping Opinion received in March 2021:
“there is a need to understand potential impacts holistically at a wider ecosystem scale rather than via the standard set of discrete individual receptor assessments. This assessment should focus on potential impacts across key trophic levels particularly in relation to the availability of prey species. This will enable a better understanding of the consequences (positive or negative) of any potential changes in prey distribution and abundance from the development of the wind farm on seabird and marine mammal (and other top predator) interests and what influence this may have on population level impacts.”
- NatureScot’s Scoping advice (December 2021) recommended that “further discussion is undertaken through the road map process to agree a suitable approach, including consideration of the upcoming OWEC PrePared project that is due to commence in 2022 and completed in 5 years”. The Ecosystem approach was discussed at the Berwick Bank Wind Farm Ornithology Road Map Meeting 4, on 31 January 2022, “The assessment should consider potential attraction to the turbine bases and how supporting processes are likely to change (e.g. physical processes). All of these dynamics should be considered through a climate change filter”.
Figure 1.1: Site boundaries for all consented and proposed wind farms currently within the Outer Firth of Forth.
- This report aims to review current literature and research on the impacts of climate change, commercial fisheries, offshore wind development, avian flu, invasive non-native species (INNS) and marine litter, which have been regarded as important threats to seabirds (Dias et al., 2019). More specifically, this report aims to:
- summarise the status of seabirds in the Forth of Tay region, and more widely across Scotland and the North Sea;
- describe the food web and associated trophic levels in the Proposed Development area;
- provide a literature review of likely significant effects on seabirds at an ecosystem-wide scale, including direct and indirect effects from climate change, commercial fisheries and offshore wind energy;
- provide a literature review of how climate change may impact seabirds in the future; and
- discuss the potential role of offshore wind energy in tackling climate and biodiversity emergencies.
- Following advice in the Scoping Opinion from MS-LOT (4 February 2022), this approach was agreed with NatureScot, RSPB Scotland and MSS through the Ornithology Road Map process (Road Map 5; Technical Appendix 11.8). This appendix reviews and presents available evidence primarily for the following seabird species, as they are relatively abundant in area of the Proposed Development and are sensitive to potential impacts from the Proposed Development as determined in volume 2 chapter 11:
- northern gannet (Morus bassanus, hereafter ‘gannet);
- common guillemot (Uria aalge, hereafter ‘guillemot’);
- herring gull (Larus argentatus);
- black-legged kittiwake (Rissa tridactyla, hereafter ‘kittiwake’);
- lesser black-backed gull (Larus fuscus);
- Atlantic puffin (Fratercula arctica, hereafter ‘puffin’); and
- razorbill (Alca torda).
2. Introduction
2. Introduction
- Climate change is leading to environmental changes at all scales through rising global sea level, increasing frequency of severe weather events, and warming oceans (Walther, 2010). This is affecting the abundance and diversity of communities at all trophic levels, with potentially catastrophic consequences for some species and communities, including seabirds. A recent global assessment of threats to seabirds concluded that invasive non-native species (INNS), fisheries bycatch and climate change are the top three threats affecting seabirds globally (Dias et al. 2019). Energy production and mining, which included offshore wind was ranked ninth of 18 threats.
- In the UK, climate change is attributed to a significant decline in breeding abundance of seabirds by 20-30% since the early 1990s (Mitchell et al. 2018) which have primarily been attributed to direct (mortality due to extreme weather) and indirect (changes in prey availability) impacts (Mitchell et al. 2020).
- Rising energy demand from global markets in combination with the climate crisis has led to the rapid growth of the renewable energy sector, with renewable energy contributing to 22% of all energy consumed in Europe in 2020 (Eurostat, 2022). Offshore wind farms are an effective way to produce renewable energy, with the EU Offshore Renewable Energy Strategy setting targets of an installed capacity of 60GW from offshore wind in Europe by 2030 (European Commission, 2020). The UK has some of the best wind resources in Europe, contributing to it becoming one of the fastest growing renewable energy industries in the UK (DECC, 2011).
- Wind energy is a key renewable energy resource for Scotland, and it is a priority in the ‘Securing a green recovery on a path to net zero: climate change plan 2018–2032 – update’ to achieve delivery of greenhouse gas (GHG) emission reduction targets (Scottish Government, 2020a; Scottish Government, 2021). Carbon savings from offshore wind electricity generation compared to the same electricity produced from natural gas is on average 143 ktCO2e per wind turbine, which is equivalent to the CO2e emitted from approximately 70,000 UK petrol cars annually (ORE Catapult, 2021).
- Scotland has ambitious targets to reduce GHG emissions; the current Scottish climate change plan 2018-2032 (Scottish Government, 2020a) sets targets to reduce emissions by 75% by 2030 (compared with 1990) and to reach net zero by 2045, which has been written into law in the Climate Change (Scotland) Act 2009 (as amended by the Climate Change (Emissions Reduction Targets) (Scotland) Act 2019). These targets are also in line with Scotland’s commitments under the 2015 Paris Agreement to limit global average temperature increases to 1.5 degrees Celsius or less. The Scottish energy strategy sets a target for the equivalent of 50% of the energy for Scotland’s heat, transport and electricity consumption to be supplied by renewable sources by 2030 (Scottish Government, 2017; Scottish Government, 2021). Scotland’s National Marine Plan calls for the sustainable development of offshore wind and other renewable energy sources in most suitable locations, for both economic benefit and to achieve decarbonisation targets of 50g CO2/kWh by 2030 (Scottish Government, 2015).
- The Scottish Government’s sectoral marine plan for offshore wind energy supports the development of between 8GW and 11GW of offshore wind capacity by 2030 (Scottish Government, 2020b). The Scottish Government's energy and offshore wind policies are further discussed in volume 1, chapter 2.
- Offshore wind farms can affect seabirds through direct impacts, such as collisions, displacement, and barrier effects, as well as indirectly through impacts on the availability of their prey. These add to existing pressures from climate change and other threats. However, there is a balance to be struck between the potential impacts from the increasing number of offshore wind farms and reduced climate change impacts from a reduction in carbon emissions.
3. Status of seabirds
3. Status of seabirds
- Seabirds are mobile and generally wide-ranging species, able to exploit a range of environments from tropical to polar latitudes (Harrison et al., 2018; Dias et al., 2019). They play a key role in marine ecosystems and can indicate the health of an ecosystem. Globally, the status of seabirds is unfavourable, with the International Union for Convention of Nature (IUCN) ranking many species as critically endangered, endangered or vulnerable (BirdLife International, 2018), with approximately 50% of seabirds estimated as declining worldwide (Dias et al., 2019). Determining the status of global seabirds is challenging since they are generally highly mobile and transient (Dunn et al., 2019).
- Currently, 25 species of seabird breed around the UK (Mitchell et al., 2004). The Seabird 2000 census estimated over 8 million breeding seabirds were present around Britain and Ireland per year, although evidence of widespread declines in productivity (number of chicks fledged per pair) have since emerged which may be driving declines in breeding population size (JNCC 2022a). Data from the most recent census, Seabirds Count, should be available in 2023 (JNCC 2022b).
- Scotland hosts internationally important populations of seabirds (Mitchell et al., 2004), supporting an estimated 45% of Europe’s breeding seabirds (Forrester et al., 2007). Many species visit seasonally, migrating to exploit temporarily available food resources and advantageous weather conditions (Forrester et al., 2007). Approximately 58% and 46% of the northeast Atlantic and world gannet populations, respectively, are present in Scotland (Murray, Harris and Wanless, 2015) and 11% and 43% of Europe’s kittiwake and guillemot populations respectively are estimated to be present around the Scottish coast (Mitchell et al., 2004; Marine Scotland, 2020; JNCC, 2021a). Population estimates for puffin from the last UK seabird census estimated Scottish populations make up approximately 40% of the GB population (Mitchell et al., 2004; JNCC, 2021a).
- There has been an estimated 70% decline in worldwide monitored seabird populations between 1950 and 2010, which can likely be attributed to human interference and industrialisation (Paleczyny et al., 2015). The UK Marine Strategy Assessment (2019) described UK breeding seabirds as having ‘not achieved Good Environmental Status’, with several species reported as undergoing frequent and widespread breeding failure. Rates of decline were highest in the early 2010s, attributed to limited food availability and the presence of INNS species (Marine Scotland, 2020). Population declines have been observed in breeding seabirds in Scotland between 1986 – 2018 ( Figure 3.1 Open ▸ ).
- In the Marine Assessment of the Forth and Tay region, abundance and breeding success have shown a degree of stability over the period 2011 – 2018, although there were some concerns around kittiwake and shag populations (Marine Scotland, 2020; Figure 3.2 Open ▸ ).
- The abundance of gannets is increasing, including at Bass Rock, the closest colony to the Proposed Development, where the population is estimated to have increased by 53% between the census in 2003-04 and 2014 (from 49,098 to 75,259 individuals) (JNCC, 2021b). During baseline surveys, gannets were abundant within the Proposed Development area, especially during the breeding season (See Appendix 11.1: Baseline Ornithology Technical Report) and have been commonly observed in other studies more widely in the outer Firth of Forth area (Camphuysen et al., 2004; Kober et al. 2010, 2012; Lane et al. 2020). The majority of gannet within the Proposed Development area are likely to originate from Bass Rock and other nearby islands, whose colonies are collectively protected under Forth Islands Special Protection Area (SPA) (See Appendix 11.4). Gannet were not assessed in the Forth and Tay Marine Assessment 2020 but are thought to have increased by 33% in Scotland between 2003 and 2015 at an average rate of 2.9% per annum (Murray, Harris and Wanless, 2015).
- Guillemots are one of the most abundant seabird species in the northern hemisphere (JNCC, 2021c), and were the most abundant seabird species observed during baseline surveys at the Proposed Development (See Appendix 11.1). Overall, guillemot populations in Scotland are estimated to have increased in the last decade (JNCC, 2021c). According to the Forth and Tay Marine Assessment, guillemot populations have remained stable (Marine Scotland, 2020). The closest colonies to the Proposed Development, St Abb’s Head NNR (+5% to 42,905 individuals in 2018), Farne Islands (+103% to 64,042 individuals in 2019) and Fowlsheugh (+12% to 69,828 individuals in 2018), have all increased in population size since the 2000 counts (JNCC, 2021c). The majority of guillemot within the Proposed Development area are likely to originate from these colonies (See Appendix 12.4).
- The Firth of Forth supports one of the largest coastal roosting populations of herring gulls in Scotland and supports a significant population of lesser black-backed gull (NatureScot, 2020). The majority of individuals of both species from baseline surveys are likely to originate from the nearby Forth Islands SPA (See Appendix 12.4), as well as potentially from nearby towns and villages which gulls may use for nesting and foraging (Balmer et al., 2013; Rock and Vaughan, 2013). The number of birds nesting in these urban environments is not well known in the UK (JNCC, 2021d; JNCC, 2021e), although they may constitute approximately 8% and 17% of herring gull and lesser black back gull populations, respectively, based on Seabird2000 counts and additional data from national authorities (Calladine et al., 2006). According to the Forth and Tay Marine Assessment, the populations of both herring gull and lesser black-backed gull have remained stable during the assessment period (Marine Scotland, 2020) although herring gull populations in Scotland are estimated to have declined significantly since the Operation Seafarer census in 1969-70 (JNCC, 2021d; JNCC, 2021e).
- Kittiwake populations were assessed to have been stable between 2011 and 2018; however, their abundance is also estimated to be less than 50% of that recorded in the 1990s (Marine Scotland, 2020). Additionally, monitored colonies close to the Proposed Development have shown a decline since the Seabird 2000 census (Fowlsheugh -25% to 14,039 individuals in 2018; St Abbs Head NNR -58% to 4,651 individuals in 2019; Farne Islands -24% to 4,402 individuals in 2019; JNCC, 2021f). The majority of kittiwake within the Proposed Development area are likely to originate from these colonies (See Appendix 12.4).
- Puffin populations are overall estimated to have increased between the 1969-79 Operation Seafarer census and the Seabird 2000 census (1998-2002). However, between the Seabird 2000 census and the most recent counts in 2017/2018, the populations at the Isle of May and the Farne Islands are estimated to have declined by 43% to 39,200 apparently occupied burrows and by 21% to 43,753 apparently occupied burrows, respectively (JNCC, 2021g). The majority of puffin within the Proposed Development area are likely to have originated from these colonies (Appendix 12.4).
- The east coast of Scotland supports several large breeding colonies of razorbill. Razorbill populations are assessed to be increasing in Scotland (JNCC, 2021g), and within the Forth and Tay region ( Figure 3.2 Open ▸ ; Marine Scotland, 2020). One of the largest population increases since the Seabird 2000 census was at Fowlsheugh, where an increase of 121% was recorded in 2018 (JNCC, 2021g). The majority of razorbill within the Proposed Development area are likely to have originated from this colony (Appendix 12.4).
- In October 2021, a new strain of pathogenic avian influenza (HPAI) was identified in the UK. Originally recorded in domestic birds and poultry, it has more recently spread to seabird colonies around Europe. Infections have been recorded in several seabird species around the UK, including great skua Stercorarius skua, gannet, guillemot and kittiwake. The full extent of the outbreak upon seabird populations is currently unknown, as discussed in Section 5.4.
Figure 3.1 Breeding seabird relative abundance from 1986 to 2019. The dashed line shows the 95% confidence limits (NatureScot, 2018).
Figure 3.2 Summary of seabird status in the Forth and Tay from Scotland’s Marine Assessment 2011 - 2018 (Marine Scotland, 2020). Blue circle = some concerns, green square = few or no concerns, 3 stars shows that the underpinning data give high confidence.
4. Seabirds and the food web
4. Seabirds and the food web
- Trophic levels describe the hierarchal levels which organisms occupy in the food web. Primary producers, such as phytoplankton and seaweed, comprise the lowest trophic levels in marine food webs. They are consumed by primary consumers (herbivores) such as zooplankton, some crustaceans (e.g. copepods) and molluscs (e.g. clams, snails, mussels). Secondary consumers (carnivores or omnivores) such as fish larvae, herring Clupea harengus and lesser sandeel Ammodytes marinus (hereafter ‘sandeel’), and some crustaceans (e.g. crabs, shrimp) feed on primary consumers and primary producers. These species support tertiary consumers, including medium fish and cephalopods (e.g. octopus and squid species). Seabirds, along with marine mammals, large fish and elasmobranchs (sharks, skates and rays), are the top predators of the natural marine food chain. An example of a marine food chain which illustrates the interactions between the different trophic levels is presented in Figure 4.1 Open ▸ .
Figure 4.1 Significant interactions modelled between functional groups and drivers (from Lynam et al., 2017).
- Typically, the marine environment follows a ‘wasp-waist’ trophic structure, where mid-trophic level species have lower diversity, compared to high diversity in both high and low trophic levels. These mid-trophic level species play an important role in ecosystem functioning (Rice, 1995). In the North Sea, the main prey groups which dominate the mid-trophic level are planktivorous fish such as sandeel, herring, sprat, Norway pout and juvenile gadoid fish, all of which are founded within the Proposed Development area (see volume 2, chapter 9). These species link the lowest trophic levels to the highest (Mackinson and Daskalov, 2007; Fauchald et al., 2011; Lynam et al., 2017). Sandeel, sprat and herring are the most important prey species for many seabirds during chick-rearing in the North Sea, including puffin, razorbill, shag and kittiwake (Wanless et al, 2018).
- The North Sea is one of the most anthropogenically impacted marine ecosystems (Halpern et al., 2015; Emeis et al., 2015). Small, shoaling forage fish in mid-trophic levels experience top-down pressure from commercial fisheries, as illustrated in Figure 4.1 Open ▸ , whilst bottom-up processes driven by temperature have dominated changes to planktonic groups since the 1960s. These pressures propagate up and down the food chain, with mid-trophic fish linking the pressures on the upper and lower trophic levels ( Figure 4.1 Open ▸ ; Lynam et al., 2017). Monitoring seabird populations can therefore provide insight not only into the top-down pressures, but also into the bottom-up pressures such as climate change, and changes to fisheries, making them a good indicator of ecosystem health (Church et al., 2019; Ramos and Furness, 2022).
- Prey availability is one of the most important controls of species abundance and distribution in the higher trophic levels, including seabirds (Lynam et al., 2012; Mitchell et al., 2020). Reduced prey availability and changing prey distribution means that seabirds may have to forage further for food. For example, Fayet et al. (2021) compared the foraging costs in puffin populations in the north-east Atlantic. They found that puffins from declining populations in southern Iceland and north-west Norway had the greatest foraging ranges and least energy-dense diet. Low prey availability close to the colonies, potentially resulting from climate or commercial fisheries effects, is also amplified by increased intra-specific and inter-specific competition which forces birds to forage further from their colonies (Fayet et al. 2021).
- Diet and foraging behaviour determine the extent to which seabird species can respond to changing prey availability. Generalist species, such as gulls, which feed on a wide range of prey types will be more resilient to changing prey availability than more specialist species such as kittiwake which are surface feeders which predominantly prey on small fish (Furness and Tasker, 2000). Water column feeders, such as auks, forage from the surface to the seabed (depending on water depth) and can feed on both pelagic and demersal fish species, as well as invertebrates such as squid and zooplankton. Surface feeders, including kittiwake and terns, are restricted to prey available within the upper 1-2m of the sea surface, such as small fish, zooplankton and other invertebrates. Therefore, changes to prey distribution within the water column resulting from changes to stratification or temperature, for example, will affect surface feeding species differently to water column feeding species.
- This has been demonstrated in the North Sea, where almost 50% of surface feeding seabird species exhibited widespread breeding failures between 2010 and 2015; compared with only two of the eight-water column feeding species assessed ( Figure 4.2 Open ▸ ; OSPAR, 2017; Mitchell et al., 2018). Typically, seabirds that feed within the water column are better able to cope with changes in prey availability than surface feeding species, as explained above (Mitchell et al., 2020). This is likely to be linked to changes in the availability of small fish species (such as sandeel and sprat species) which are the predominant prey of surface feeding species such as kittiwake (Frederiksen et al., 2005; Carroll et al., 2017). A summary of the typical feeding strategy and prey of key seabird species for the Proposed Development has been outlined in Table 4.1. Plunge divers dive into the sea from a height to catch prey, whereas pursuit divers dive and can then swim underwater in pursuit of prey.
Figure 4.2 Proportion of seabird species (%) in the North Sea not showing widespread breeding failure, 1992 – 2015. The red horizontal line denotes the target threshold of 75 % or more. OSPAR, 2017)
- The availability of sandeels has been correlated with the breeding success and adult survival of kittiwakes (Frederiksen et al., 2004, 2008a; Carroll et al., 2017). Adult kittiwakes eat mostly older (1+ year group) sandeels during April and May; switching to juvenile (0-year group) sandeels in June and July during chick rearing (Lewis et al., 2001). This correlates with the annual cycle of sandeels. The 1+ year group (sandeels hatched prior to the current year) are active in the water column during spring. Once they have accumulated enough lipids they bury themselves in the sand, usually in June-July, and live off their stored lipids during the winter. The 0-year group (young of the year) sandeels are available from June onwards following metamorphosis from larvae into juveniles, and prior to burying themselves to overwinter (Wright and Bailey, 1996). However, density dependence also influences sandeel recruitment, and the biomass of the sandeel stock tends to be driven by occasional especially good years (ICES, 2017). In sandeel stocks with low fishing mortality, years with high stock biomass tend to show low recruitment, whereas high recruitment is more likely when adult stock biomass is lower (ICES, 2017, Lindegren et al. 2018). Both climate change and commercial fisheries are implicated with a reduction in sandeel abundance, which may contribute to kittiwake declines (Carroll et al., 2017).
- In the western North Sea between 1973 and 2015, the diet of chick-rearing kittiwakes, puffins, razorbills and shags was predominantly comprised of sandeels (Wanless, et al., 2018). Clupeids (sprat and herring) were the second-most important prey species, however these rarely exceeded 10% of the food biomass per year. Juvenile gadids were another important prey species (1 - 10% biomass) for these seabird species in some years (Wanless, et al., 2018).
- For guillemots, sandeels were the predominant prey until the late 1990s, when a shift to sprat (93%) and herring (7%) was observed (Wanless, et al., 2018). Between 1982 and 2019, sandeels were largely confined to the early part of the chick period as they have declined (Harris et al., 2022). A trend towards more sprat and herring have also been observed since the mid-2000s in razorbills and kittiwakes during chick-rearing, though sandeels are still the dominant prey (Wanless et al., 2018). Sprat feed and spawn repeatedly through spring and summer in coastal and offshore waters, and so are available for a wider period (MCCIP, 2018). Gannet predominantly feed on pelagic fish such as mackerel and sandeel, and also feed on fisheries discards, however the introduction of bans on discards may reduce the availability of this food source (Le Bot et al., 2019).
- Gull species, such as herring gull and lesser black-backed gull are able to feed on a diverse range of prey and food from both natural and anthropogenic sources (Table 4.1). In the south-eastern North Sea, faecal samples revealed that both lesser black-backed gulls and herring gull diets were predominantly composed of bivalves and crustaceans (Kubetzki and Garthe, 2003). A decline in herring gull abundance has been observed in Scotland since the 1969-70 National Census, and lesser black-backed gull populations have strongly fluctuated, which has been associated with changes in waste management such as covering refuse tips, and a reduction in fisheries discards (Burthe et al., 2014; Foster, Swann and Furness, 2017; JNCC, 2021d; JNCC, 2021e; Tyson et al., 2015); this may be evidence of the over-reliance of these species on these food sources. Foraging at landfills can also increase the risk of disease and mortality from Clostridium botulinum infection (Coulson, 2015).
5. Anthropogenic activities causing direct effects on seabirds
5. Anthropogenic activities causing direct effects on seabirds
5.1. Climate change
5.1. Climate change
- Climate change and severe weather events are one of the biggest threats to UK seabird populations (IPCC, 2014; Dias et al., 2019). Direct effects on seabirds which arise from climate change include storms, heavy rainfall events, flooding of nesting sites and temperature extremes.
- Strong winds and heavy rainfall during the breeding season can chill eggs, kill nestlings, and prevent adults foraging adequately to feed chicks, which can result in widespread breeding failures (Mallory et al., 2009; Mitchell et al., 2020). Flying and diving under high wind speeds can also impact body condition, by requiring greater energy expenditure (Kogure et al., 2016), and reducing foraging success. Storm conditions can reduce the availability of prey at the water surface and can increase water turbidity which reduces the ability of visual hunting seabirds, such as cormorants and shags, to find food. This can contribute to mass mortality events, which are further discussed in Section 6.4.
- Gannet may be able to take advantage of strong winds during adverse weather by modulating their flight height, giving them some resilience to storms (Lan, Spracklen and Hamer, 2019). For guillemot, the negative demographic impact of storms and adverse weather may be buffered to some extent by their large population size (Johnson et al., 2021).
- Seabirds are typically either ground nesting (e.g., herring gull, lesser black-backed gull, Arctic tern), burrowing (e.g., puffin), or cliff nesting (e.g. gannet, guillemot, kittiwake, razorbill). Flooding from heavy rain and storm surges can flood or wash away nests, and high winds can damage or destroy nests. Additionally, rising sea levels may reduce the habitat availability of low-lying nesting species, such as terns (Ratcliffe et al., 2008), with an estimated mean sea level increase of 4cm (likely range between 1 and 7cm) in 2018, when compared with the 1981-2000 average in the Forth and Tay region (Marine Scotland, 2020). For example, low productivity of Scotland’s puffins in 2007 has been partially attributed to unprecedented rainfall which flooded their burrows (Newell et al, 2013). An estimated reduction of 15% of kittiwake nests and 28.5% of razorbill nests was caused by a single storm in one year at the Isle of May (Newell et al., 2015). Gull species, such as herring gull and lesser black-backed gull, are able to utilise urban environments for nesting, which may give them resilience against any reduction in available nesting habitat (Balmer et al., 2013; Rock and Vaughan, 2013).
- Abnormally hot weather can cause stress to seabirds through issues with heat dissipation. For example, great skuas have been found to spend more time bathing in higher temperatures, meaning less time is spent foraging and tending to chicks (Oswald et al., 2008). Diving species may have significant heat loss to the water during diving, however this also generates metabolic heat (Oswald and Arnold, 2012). Puffins are thought to use their relatively large bills to dissipate excess metabolic heat, and so may be more resilient to hot weather than other species (Schraft, Whelan and Elliott, 2019). When combined with indirect effects of climate change, such as reduced prey availability, this can have critical effects on chick survival (Oswald et al., 2008; Hakkinen et al., 2022).
- There is a great degree of uncertainty in climate models for the North Sea, but there is potential that the magnitude and frequency of extreme weather events could increase as a result of climate change in the North Sea (Rahmstorf and Coumou, 2011, Wolf., Woolf and Bricheno, 2020). This would be likely to present population-level consequences for many seabird species, as outlined above (Frederiksen et al., 2008b; Louzao et al., 2019).
5.2. Commerical fisheries
5.2. Commerical fisheries
- Commercial fisheries and seabirds have the potential to interact due to spatial and temporal overlap between foraging ranges and fishing activity (Žydelis et al., 2011; Lewison et al., 2014). In the vicinity of the Proposed Development, and in the wider Forth and Tay area, the dominant fishing types are demersal trawling, predominantly for Nephrops, followed by creeling for lobster and crab and dredging for scallops (volume 2 chapter 12; Marine Scotland, 2020).
- Bycatch (the incidental capture of seabirds by commercial fisheries) has significant impacts on seabirds globally (Tasker et al., 2000; Dias et al. 2019). Primarily, fishing gear in which seabirds are most likely to be bycaught are those involving fixed nets or longlines (Anderson et al., 2011; Žydelis et al., 2013). Annually, an estimated mortality of 400,000 birds is predicted to occur in gillnet fisheries (Žydelis et al., 2013) and an estimated hundreds of thousands in longline fisheries (Anderson et al., 2011). Whilst these fishing types are uncommon in the Forth and Tay, seabirds from the Proposed Development area may be exposed to these fisheries when foraging further offshore, or during migration.
- Bycatch from trawling can result from diving birds, such as guillemot, becoming entangled with trawls, or at the surface where seabirds can be struck by cables which are used to tow and monitor the equipment, thus injuring or drowning the bird (Løkkeborg, 2011). Commercial fisheries bycatch has been previously linked with a decline in population abundance in guillemot (Johnson et al., 2021).
- With commercial fishing effort increasing year-on-year (FAO, 2020), bycatch risk is likely to increase due to increased potential for interaction between industry and birds. Nearly 30% of seabird species analysed by Dias et al. (2019) were predicted to be affected by bycatch, with the number of globally threatened species affected increasing from 40 to 50 since 2010.
- Several British seabird species are at risk of being captured incidentally, with guillemots, razorbills and diver species being most susceptible (Bradbury et al., 2017; Larsen et al., 2021). Seabird foraging strategy affects susceptibility per gear type, with pursuit divers (e.g. guillemots) and plunge divers (e.g. gannets) more likely to be bycaught in gillnets rather than surface feeders such as kittiwakes (NatureScot, 2018). A study of Danish fishing boats operating within the North Sea found seabirds were more susceptible to bycatch in the winter compared to in the breeding season, potentially due to there being less prey available during winter, so seabirds come closer to the nets to secure food (Larsen et al., 2021).
- Recently, preliminary estimates of total annual seabird bycatch mortality in the UK have been calculated for 2016 and 2017, based on data from static net, longline and midwater trawl fisheries (Northridge, Kingston and Coram, 2020). Annually, 1,800-3,300 guillemots and 2,200-9,100 fulmars were estimated to be incidentally captured across the UK waters, predominately in static and longline fisheries, respectively ( Figure 5.1 Open ▸ ). However, true estimates may be higher than this as only UK vessels were included in the analysis (Northridge, Kingston and Coram, 2020). Demersal trawls, ring nets and pots were also not included in these analyses due to relatively low observation levels.
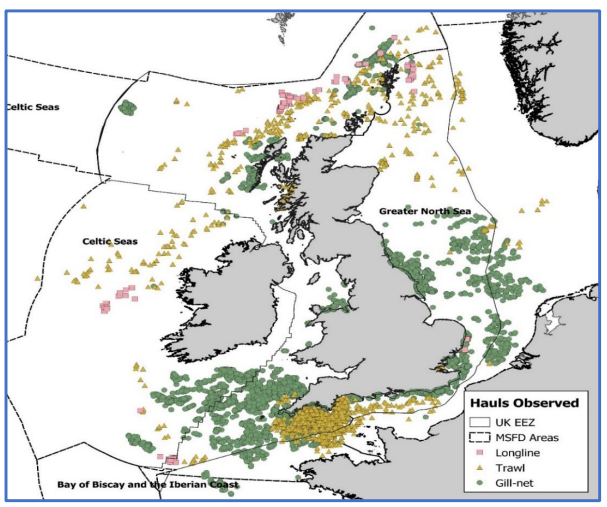
Figure 5.1 Observed hauls (left) and observed seabird bycatch by species (right) between 1996 and 2018 (Northridge, Kingston and Coram, 2020). Bycatch observed from 21,261 hauls across 3,455 trips in this period.
- Fisheries discards can also provide an alternative food source to generalist feeders, such as gulls and gannets, when natural prey species are not available, which may benefit some seabirds. However, since 2019 in the EU (European Commission, 2019) and the UK (Marine Management Organisation, 2019), landing obligations are being enforced which eliminates commercial fisheries discards, which will likely have reduced the availability of this food source (Uhlmann, Ulrich, and Kennelly, 2019; Le Bot et al., 2019), however the impact of this ban on seabirds is not yet known.
5.3. Offshore wind energy
5.3. Offshore wind energy
- The potential for direct adverse effects on seabirds arising from the presence of offshore wind farms is broadly determined by overlap between species habitat and placement of wind farm arrays. The predominant direct effects acting on birds from the presence of offshore wind farms are related to collision, barrier effects and displacement (Gibson et al., 2017).
- Collision of seabirds with offshore wind farms may result in direct mortality or lethal injury (Desholm and Kahlert, 2005). The risk of collision varies between species and is affected by behaviour, population size, time of year, weather and wind farm design (Gill, 2005; Drewitt and Langston, 2006). Seabird species most at risk of collision with offshore wind farms are those which predominantly fly at the height of the wind turbine blades (Furness et al., 2013; Johnston et al., 2014). Eight seabird species present within the Proposed Development were considered to be vulnerable to collision based on the wind turbine height (Furness et al., 2013): kittiwake, herring gull, lesser black-backed gull, gannet, Arctic tern, common tern, little gull and great skua. Auks and fulmar were not considered vulnerable to collision as they generally fly at low altitudes, well below the minimum height of wind turbine rotor blades (Cook et al., 2012; Johnston et al., 2014; Jongbloed, 2016).
- For the Project, collision risk was assessed for kittiwake, herring gull, lesser black-backed gull, gannet, Arctic tern, and great skua (Appendix 11.3). For all species, the worst-case collision impacts were predicted for the smallest 14 MW wind turbine and the estimated number of annual collisions was highest for kittiwake. The consequences of collisions on seabird populations at SPAs are considered in Appendix 11.6. To reduce the collision risk to seabird species, Project design measures have been included, such as increasing the minimum lower blade tip height to 37m above LAT.
- Collision risk has been studied at a variety of offshore wind farms (Christensen et al., 2004; Blew et al., 2008; Krijgsveld et al., 2011; Leopold et al., 2013; Skov et al. 2018), however, studies reliant on carcass recovery at sea are inherently biased because carcasses drift, sink and can be scavenged. In offshore wind farms, models (e.g. Band, 2012) are used to predict estimated numbers of collisions for impact assessment purposes. Collision is more likely to occur if seabirds fail to avoid wind farms, although there is growing evidence of micro-, meso- and macro- scale avoidance, by a variety of species (Cook et al., 2014a; Bowgen and Cook, 2018).
- The presence of offshore wind farms can lead to displacement (where seabirds foraging within an area are displaced by the installation and operation of a wind farm) and barrier effects (where a movement corridor for seabirds has been effectively blocked by the installation of a wind farm) for some seabirds, with the potential for energetic consequences (Humphreys, Cook and Burton, 2015).
- Species which have been observed to exhibit displacement and barrier effects in northwest Europe include guillemot, razorbill, grebes, divers and gannets, whereas gull species may be attracted to the offshore wind farms (Dierschke et al., 2016). Displacement assessments were undertaken for kittiwake, guillemot, razorbill, puffin and gannet for the Project (Appendix 11.4) and the estimates of potential mortality due to displacement modelled for the relevant SPA populations in Appendix 11.6.
- Tracks of individual birds in the vicinity of three offshore wind farms at Helgoland, North Sea indicated that 89% of gannets avoided wind farm areas (Peshko et al., 2021). Analysis of GPS tracking data at the Proposed Development estimated that only 0.7% of the home range of gannet foraging from Bass Rock, the closest significant colony, and 74% of gannet did not enter the proposed development area at all (Appendix 11.4, Annex E).
- Increased energy expenditure associated with longer trip times between breeding colonies and foraging areas as a result of displacement and/or barrier effects can adversely affect local bird populations, with the potential to cause population-level effects (Masden et al., 2010). Displacement to other areas can also increase the pressure on mid-trophic prey species, such as sandeel, sprat and herring, in the areas which seabirds might be displaced to. For migratory birds, potential barrier effects can be reduced through altering wind turbine configuration to create corridors (Krijgsveld, 2014). However, the level at which species may be affected by barrier and displacement effects is difficult to quantify, with both likely to be confounded by other population-level effects such as fluctuations in prey distributions or the presence of fishing vessels immediately outside the wind farm boundary (Votier et al., 2010; Vanermen et al., 2013).
5.4. Avian influenza
5.4. Avian influenza
- Avian influenza (commonly referred to as ‘avian flu’ or ‘bird flu’) is a virus that causes disease in birds, affecting the respiratory, digestive and/or nervous system of many species. Typically, infections are from a low pathogenic viral strain which causes mild illness. However, strains can mutate from low to high strains, which cause severe symptoms, often with high mortality rates and these strains may spread quickly causing an outbreak. The virus has become a disease of global significance due to poultry intensification creating conditions favourable for transmission of highly pathogenic strains (Gilbert and Xiao, 2008).
- In October 2021, a new strain of HPAI (H5N1) was identified in the UK. Since then, 120 further locations of infection in captive birds and poultry, have been identified across the UK, and 354 separate locations of infection across wild birds of 63 species have been identified across 76 countries worldwide (DEFRA, 2022). This has been the highest recent occurrence of HPAI in the UK with 90 cases of outbreak, compared with 28 in winter 2016/17 and 13 in 2020/21 (Lean et al., 2022). The greatest proportion of infection to date has been observed in swans, geese and ducks, and these species may form a natural reservoir of the virus. High occurrences of the 2021/22 avian flu have also been recorded in waders, gulls and auks as well as in species of birds of prey (DEFRA, 2022).
- In Scotland since October 2021, there have been HPAI (H5N1) infections recorded in several seabird species, including gannet at Bass Rock and Hermaness, Shetland (Martin, 2022) and Isle of Noss (Philip and Tyler, 2022), guillemot at St Abb’s Head (Hall, 2022) great skua at St Kilda, Fair Isle, Isle of Noss and Foula (Banyard et al., 2022; NatureScot, 2022a; Philip and Tyler, 2022) and kittiwake, great black-backed gull and terns at the Isle of May (Steel pers comm. 19 July 2022; NatureScot, 2022a). In August 2022, there had been no mass mortalities observed in Scottish tern, razorbill or puffin colonies (Philip and Tyler, 2022). However, the full magnitude of impact is currently highly uncertain.
- The effect of environmental variables on HPAI transmission and persistence is poorly understood (Gilbert et al., 2008; Lane et al., 2022). However, climate change is impacting the migration routes and distributions of birds, for example altering the timing of migration, stopover, and breeding, as well as the availability of breeding sites. This could lead to increased opportunity for transmission of HPAI between wild populations or between wild and domestic birds (Tian et al., 2014). Climatic changes may also alter the ability of the virus to survive in the environment, with potential to increase infection and transmission rates if there is longer environmental survival (Martin, Becker and Plowright, 2018).
5.5. Invasive non-native species
5.5. Invasive non-native species
- Seabird populations have been severely impacted by the introduction of mammalian predators to regions where they would not ordinarily occur (Dias et al., 2019). Brown rats, cats, and American mink are among the introduced non-native predatory mammals to Scotland that pose a serious threat to seabird colonies. Native mammals brought by humans to offshore islands, such as hedgehog, stoat, and fox, also pose a significant threat (Marine Scotland, 2020). For example, American mink have been estimated to reduce herring gull breeding success by 41% in the west Scottish islands (Coulson, 2019). During site-specific surveys of the Proposed Development, no INNS were observed (see Appendix 22, Annex 22.8).
- Tree mallow is native to coastal areas of the Mediterranean and the south-west coast of the UK, but when introduced beyond these locations it often becomes invasive, causing significant changes to vegetation communities (Van der Wal, 2006). In the Firth of Forth region, declines in puffin populations have been linked to an increase in invasive tree mallow, which can block the entrances to puffin burrows and prevent breeding. Active management has led to a decline of the plant at Craigleith from 80% coverage to less than 10% between 2010 and 2020 (Anderson, 2021a; Anderson, 2021b).
5.6. Pollution
5.6. Pollution
- Pollution in the marine environment which can affect seabirds includes oil pollution, other chemical contaminants, and marine litter such as plastics (Burthe et al., 2014). Oil spills can cause suffocation of seabirds and can disrupt feather integrity, leading to a loss of water proofing and buoyancy, and an inability to dive or fly (Troisi, Barton and Bexton, 2016). Chemical pollutants can accumulate in the tissues of predators such as seabirds, which can adversely affect survival rates and productivity (Letcher et al., 2010; Votier et al., 2005; Votier et al., 2011). For example, winter mortality of guillemot has been demonstrated to by doubled by major oil pollution incidents (Votier et al., 2005).
- Marine litter is persistent, manufactured and/or processed anthropogenic material found in the marine environment, the most common of which is plastics and fishing gear (UNEP, 2021). Between 2016 and 2020, plastic production in Europe increased by almost 10% (Plastics Europe, 2021), a proportion of which is likely to end up in the marine environment as plastic waste. After encountering marine litter, many marine species ultimately end up ingesting it or becoming entangled, which can be fatal (Good et al., 2010; Roman et al., 2019).
- The ingestion of marine litter can be detrimental by blocking or puncturing intestines and through the assimilation of harmful chemicals which leach out of items (Carey et al., 2011; Tanaka et al., 2013). Some models indicate 99% of all seabird species are predicted to ingest plastic by 2050 (Wilcox et al., 2015). One study indicated seabirds can have around a 20% chance of fatality from ingesting just one piece of marine litter, with the composition of the litter influencing probability of death (Roman et al., 2019). Some species groups are more susceptible to ingestion of marine litter due to their life history strategies, with surface feeders such as fulmars more susceptible than pursuit divers such as guillemots due to the assimilation of marine litter on the water surface (Provencher et al., 2010; Daunt and Mitchell, 2013). Smaller items such as microplastics may also release chemicals which are ingested by organisms in lower trophic levels and can bioaccumulate in high trophic level species, such as seabirds (Miller et al., 2020).
- When considered alongside climate change, the effects of marine litter can be made exponentially worse, with the potential for items to add to negative feedback loops through emission of greenhouse gas during the lifetime of items (Ford et al., 2022). Climate change also has the potential to affect marine litter by increasing flux into oceans through extreme weather events and associated run off, in addition to affecting degradation through heat and light exposure (Deng et al., 2021). Fragmentation of items and increased likelihood of small items remaining in suspension may also be more likely to occur under storm conditions, which are expected to increase due to climate change.
6. Anthropogenic activities causing indirect effects on seabirds
6. Anthropogenic activities causing indirect effects on seabirds
6.1. Climate change
6.1. Climate change
- Climate change is leading to dramatic changes in ecosystem structure, through effects on ocean temperature, water stratification and nutrient availability, leading to changes in the abundance and diversity of communities at all trophic levels, from primary producers to top predators (Walther, 2010). Effects of climate change have been identified over a variety of timescales. Short-term variability in environmental conditions impacts interactions between trophic levels and species (Howells et al., 2017). Limitations in prey availability can adversely affect top predators, with population-level changes likely to occur over longer timescales, propagating up trophic levels with prolonged exposure (Frederiksen et al., 2006; Howells et al., 2017).
- Indirect effects on seabirds are primarily mediated through prey availability, diversity and quality, related to changes in primary producers and directly to key prey in the mid-trophic levels (Lynam et al., 2017; Mitchell et al., 2020). Lynam et al (2017) statistically modelled complex patterns of trophic control in the North Sea, driven by fishing mortality and climatic variables. The average success of seabird reproduction was linked to climate change through temperature effects on primary production and primary consumers (e.g. plankton species) and pressure from commercial fisheries, and the subsequent effects on forage fish species ( Figure 4.1 Open ▸ ). Hakkinen et al. (2022) ranked climate change threats to seabirds in western Europe and concluded reduced prey availability was the greatest threat. Access to enough prey, with sufficient nutrient quality, at the right time and location is more critical than the absolute abundance of prey (ICES, 2015; Mitchell et al., 2020). Changes to prey availability and quality has potential consequences over moderate to long timescales following mismatch in life-history events between species (Visser and Gienapp, 2019). Other indirect effects include range shifts, which may be catastrophic for nest-building species where new environments may have reduced nesting materials or suitable habitat, changes in interactions with predators or potentially harmful diseases and parasites (Mainwaring et al., 2016).
- In the Forth and Tay region, the SST has increased by 0.05°C per decade on average since 1870, and an increase of 0.21°C per decade between 1988 and 2017, suggesting that warming has accelerated in the last 30 years (Marine Scotland, 2020). An assessment of the vulnerability of seabirds in the Forth and Tay region demonstrated negative correlations between SST and abundance, adult survival and/or productivity of 57% of the investigated bird species in the area (Burthe et al., 2014). This was predominantly linked to the impact of climate change on the lowest trophic levels, such as plankton, and the subsequent effects on seabird prey availability.
6.1.1. Prey availability, quality and distribution
- Declines in abundance and quality of mid-trophic level-species have been linked to multiple climate change-related factors, such as increasing SST, changes in stratification and alterations in the North Atlantic Oscillation (Johnston et al., 2021). Generally, scenarios with colder conditions were expected to benefit seabird populations through the increase in primary consumers (such as small copepods and other zooplankton) and therefore sandeel abundance, meaning that SST increases related to climate change may be detrimental to the abundance of sandeels and similar mid-trophic species (Lynam et al., 2017).
- For example, in the North Sea, kittiwake over-winter survival has been observed to be lower following winters with a higher SST, with lower breeding success in the following summer which is likely related to reduced sandeel availability (Frederiksen et al., 2004, 2005, 2007). However, in the Celtic Seas region, where kittiwake diets are predominantly composed of sprat and herring, no relationship has been observed between SST and kittiwake breeding success, evidencing the vulnerability of North Sea kittiwake populations to sandeel populations declines (Lauria et al., 2013; Cook et al., 2014b).
- Guillemot population collapse and recovery in Norway has been linked to climate mediated bottom-up impacts on prey species capelin Mallotus villosus, herring and cod (Erikstad et al., 2013), and breeding success has been repeatedly cited as correlating with prey abundance of sprat in the Baltic Sea (Kadin et al., 2012), sandeel, within the North Sea (Rindorf, Wanless and Harris, 2000; Frederiksen et al., 2006; Burthe et al., 2012); cod, in the Norwegian Sea (Barrett and Erikstad, 2013) and herring, in the Celtic and Norwegian Seas (Lauria et al., 2012; Barrett and Erikstad, 2013). Puffin fledgling success has been negatively correlated with SST on the Isle of May (Burthe et al., 2012) and St Kilda (Harris, Murray and Wanless, 1998) relating to bottom-up influences on sandeel abundance (Frederiksen et al., 2013).
- Sandeels are an important trophic link between plankton and predators in North Sea ecosystems; however, climate driven changes to phytoplankton and zooplankton (further discussed in Section 6.1.2) and commercial fishing (further discussed in Section 6.2) have led to declines in the abundance and nutritional quality of these species and other small planktivorous fish since 2000 (Macdonald et al., 2015; Clausen et al., 2017; Wanless et al., 2018; MacDonald et al., 2019). This is correlated with community-wide declines in sandeel abundance in the diets of chicks of auks, shags and kittiwakes observed over 30 years at the Isle of May (Wanless et al., 2018), and sandeels becoming largely confined to the early part of the chick rearing period of guillemot (Harris et al., 2022).
- At the Isle of May between 1973 and 2015, it is estimated that the energy content of sandeels decreased by approximately 70% and 40% for 0 and 1+ year groups, respectively, indicated by a decrease in the mean length-at-age of sandeel prey (Wanless et al., 2018) which has been correlated with the productivity of guillemot, kittiwake and razorbill (Burthe et al., 2012; Wanless et al., 2018). The breeding productivity of shag, kittiwake, puffin and guillemot on the Isle of May have also been associated with the mean size of 1-group sandeels (Frederiksen et al., 2006). In 2004, the calorific densities of sprat and sandeel were less than 25% of that expected compared with previous years, which is thought to be the primary cause of guillemot breeding failures in that year (Wanless et al., 2005). Similar observations for a range of marine birds and mammals have given support to the ‘junk food’ hypothesis which states that the energy density of low-lipid prey is inadequate to meet the energetic demands of breeding, even if such prey are abundant (Piatt and Anderson 1996; Merrick, Chumbley and Byrd, 1997; Rosen and Trites 2000; Litzow et al. 2002).
- Like sandeel, Atlantic mackerel Scomber Scombrus population declines have been linked with SST, which may have an adverse effect on their growth, recruitment and migration (Jansen and Gislason, 2011). Jansen et al. (2012) also observed a decline in mackerel larval abundance in the North Sea since the 1970s. Whilst this cannot necessarily be attributed to climate change, mackerel eggs have shown sensitivity to seawater temperature (Mendiola et al., 2007). Burrows et al. (2019) considered the temperature affinity of bottom-dwelling fish species. They demonstrated that, with the warming climate, there has been a shift towards warm-water species such as lesser-spotted dogfish, hake and horse mackerel and away from cold-water species such as Atlantic cod, Atlantic herring and sprat) across the North Sea. Seabird species which are able to exploit these warm-water species may have higher resilience to changes in prey availability associated with climate change.
- The resilience of seabirds to changing prey availability may be affected by their feeding strategy (Burthe et al., 2014). Guillemot’s pursuit diving behaviour, allowing them to forage at a range of depths, may provide resilience to fluctuations in prey availability, making them more resilient to climate change effects (Furness and Tasker, 2000; Johnston et al., 2021). Shags have been observed to diversify their diet with increased SST, associated with a decrease in the proportion of sandeels in their diets, possibly leading to consequences in population dynamics (Howells et al., 2017). Additionally, species which are able to be more generalist in their diet, such as gull species and gannets, are likely to be able to better respond to changes in prey availability, however species such as kittiwake which are more dependent on sandeels as their primary food source are likely to be more adversely affected by changes to prey availability (Burthe et al., 2014; Furness and Tasker, 2000; Carroll et al., 2017).
6.1.2. Copepod availability and distribution
- Changes in the phenology, community composition, abundance and distribution shifts are anticipated at lower trophic levels as a result of rising SST) and climate change, which could cascade through the food webs and affect the availability of seabird prey species at mid-trophic levels, and thus seabird populations (Burthe et al., 2012; Lynam et al., 2017). Off the east coast of Scotland between 1973 and 2015, slow growth was observed in sandeels which correlated with temperature and zooplankton and phytoplankton biomass, indicated by a reduction in the length and energy value (MacDonald et al., 2019). Similar trends have also been observed in herring and sprat in the west of Scotland (Hunter, Speirs and Heath, 2019), and sprat, sandeel, herring and Norway pout more widely across the North Sea (Clausen et al., 2017).
- The copepod Calanus finnmarchius constitutes more than 50% of the total copepod biomass in the North Atlantic and is an important prey species for small fish which seabirds feed on (Frederiksen et al., 2013). Frederiksen et al (2013) found a strong correlation between C. finnmarchicus environmental suitability and the distribution of kittiwakes, guillemots and puffins in the North Sea. They also found that C. finnmarchicus is adversely correlated with SST. Therefore, increasing temperatures related to climate change could see a northward distributional shift in C. finnmarchicus and contribute towards a northward shift in small pelagic fish and seabirds (Frederiksen et al., 2013). Similarly, anomalously high SSTs during the breeding season in the Northwest Atlantic have been correlated with reduced prey availability and accessibility due to the formation of a thermocline, which subsequently reduced the condition of gannet, shortened the chick rearing period (Franci et al., 2015) and reduced breeding success (Montevecchi et al., 2013).
- Similarly, northward-distribution shifts were predicted in copepod communities across the North Atlantic, by McGinty et al (2021). Models also predicted basin-wide shifts in community composition, with an increase in carnivorous species and a reduction in herbivorous species, and a decrease in body sizes of lower trophic groups as they move northward (reference). These changes may reduce energy and carbon transfer to higher trophic levels and have subsequent impacts to trophic links and ecosystem functioning (McGinty et al., 2021). This may be correlated with a northward shift in the distribution of forage fish species, such as sandeel and sprat (Burthe et al., 2012).
- Climate change is also leading to ocean acidification, by chemical processes related to increased temperatures increasing dissolved levels of carbon dioxide in seawater. Decreasing pH is affecting phytoplankton, which can inhibit shell generation of calcifying marine organisms and may impact skeletal development in larval fish, with potential consequences to forage species of seabirds (Riebesall et al., 2013). However, these impacts are difficult to predict at species and population levels due to the complexity of these food web interactions (Heath et al., 2012).
- In the Forth and Tay region, there has been limited study of plankton and assessments which have been undertaken are too short in duration to understand the local impacts on climate change on the lowest trophic levels (Marine Scotland, 2020). The Scottish Environment Protection Agency has monitored some phytoplankton (diatoms and dinoflagellates) in the Firth of Forth and the Clyde between 2010 and 2017, which estimated no change in their abundance during this period (Marine Scotland, 2020). Due to insufficient knowledge, it is difficult to predict the future of the lowest trophic levels (e.g., primary producers and primary consumers such as phytoplankton and plankton) in the Forth and Tay region, or in the Proposed Development area.
6.1.3. Mismatch in life-history events
- A further key factor in sandeel larval success is synchrony between the larval hatching times and the spring zooplankton bloom (MCCIP, 2018).
- Warming has been demonstrated to lead to earlier zooplankton blooms in higher latitudes (Greve et al., 2004; Mackas and Beaugrand, 2010; McGinty et al., 2021), resulting in a decrease in zooplankton available for sandeels to feed upon and a reduction in sandeel growth and survivorship (Réginer, Gibb and Wright, 2017). Bloom timing can also have knock-on impacts to other species, for example by introducing competition between species which did not previously overlap spatially, or in the timing of key reproductive events such as foraging.
- Increased sea temperatures are expected to lead to later larval hatch times in sandeel (Boulcott and Wright, 2008; Wright, Orpwood, and Scott, 2017), which may also have contributed to reduced sandeel size (Wanless et al., 2004). Adult sandeels feed on plankton in the spring and summer months; building up lipids to survive the winter period buried in sand when plankton production is lower. Increased temperatures lead to increased energy usage whilst overwintering, meaning less energy can be allocated to gonad development. (Boulcott and Wright, 2008; Wright, Orpwood, and Scott, 2017). Sprat feed and spawn repeatedly through spring and summer and therefore do not rely on winter energy stores and so are less sensitive to the timing of the onset of plankton production than sandeels (MCCIP, 2018), which may have contributed to prey-shifts in auks and kittiwakes from sandeels towards sprat in the North Sea (Wanless et al., 2018). However, for such prey to provide an adequate alternative to traditional prey like sandeels, they must also be of sufficient abundance, availability, and quality.
- Warming climates and SST have also been correlated with later laying dates of puffin at the Isle of May and St Kilda (Burthe et al., 2012) which also correlated with a reduction in the success of chicks (Harris et al., 1998). Delayed breeding may be a response to the timing of peak prey abundance (Durant, Anker-Nilssen, and Stenseth, 2003), however the shift towards later breeding may be insufficient, leading to a mismatch in the timing of puffin chick rearing and adequate prey quality, size and quantity and contribute to declines in chick success (Burthe et al., 2012; Harris, Murray and Wanless, 1998; Johnston et al., 2021).
6.1.4. Competition
- The change in prey availability and distribution, and in available nesting habitat in the North Sea could also increase the inter-specific (between species) and intra-specific (within species) competition in seabirds. Northward shifts of birds could see populations which previously did not overlap spatially become closer, leading to competition for prey and nesting habitat in the same area (Mainwaring et al., 2016; Sadykova et al., 2020). This may also lead to an increase in the transmission of disease and parasites between populations whose ranges may not have overlapped, historically (Mainwaring et al., 2016).
- Climate change is also affecting the distributional range of other marine predators, such as European hake Merluccius merluccius which has expanded its spatial distribution in the North Sea in correlation with temperature (Cormon et al., 2016). This may have a direct impact on its prey species Norway pout and herring, which could have knock-on food web impacts on prey availability for seabirds (ICES, 2014). Climate change and overfishing has also been linked to a global increase in jellyfish populations (Purcell, Uye and Lo, 2007). Jellyfish directly compete with planktivorous fish in the lower-mid trophic levels for food such as copepods, whilst also predating on fish larvae in some species, and provide refuge for juvenile gadoids (e.g. cod and hake), which may increase competition for seabirds and their prey (Lynam and Brierly, 2007; Lynam et al., 2017).
6.2. Commerical fisheries
6.2. Commerical fisheries
- Forage fish landings account for approximately one third of global landings of marine fish, not considering additional loss from bycatch discards (Alder et al., 2008). Fisheries can disrupt the food web by removing target species from the mid-trophic levels, such as sandeel and herring, which are important prey species for top predators such as seabirds. This can have significant indirect impacts on seabird growth, survival, foraging behaviour, chick provisioning and breeding success, by affecting the amount of prey available, the energy available per prey item and the timing of seasonal dietary shifts (e.g. Frederiksen, 2008a; Sherley et al, 2018).
- In the North Sea, annual landings of sandeels have halved since the late 1990s, when landings peaked at over one million tonnes (ICES, 2019). The fishery at the Wee Bankie sandbank, 40 km off the east coast of Scotland outside of the Firth of Forth, was closed to the sandeel industrial fishery in 2000 as part of the northeast UK sandeel closure after concerns were raised about the effect of sandeel population decreases on predators, particularly seabirds (Scottish Marine Assessment, 2021). The northeast UK sandeel fishery (ICES sandeel area 4) span across a large portion of the foraging seabirds from the Proposed Development area. Following closure, sandeel abundance significantly increased, most likely due to stronger recruitment of 0 group individuals (Furness and Camphuysen 1997; Greenstreet et al., 2010). Despite this, there are still concerns over the management of the fishery as the fishery currently operates within the foraging range of internationally important seabird species and the management plan does not include protective measures to ensure species dependent on sandeels such as seabirds or lower trophic level species can still function effectively, in addition to the effect of concentrating fishing effort into smaller areas, which can ultimately lead to the reduction of localalised sandeel stocks (Dunn, 2021).
- Kittiwakes have been found to be particularly susceptible to changes in sandeel populations caused by commercial fisheries. While the Wee Bankie fishery was in operation, a strong decline was observed in breeding success for kittiwake colonies which overlapped with the closed fishery area, including the Isle of May (Searle et al., 2022). Kittiwake populations increased by approximately 8% in this area between the closure of the fishery and 2018 (Searle et al., 2022), and the shift from 1+ to 0-group sandeels were found to occur earlier in the years following the closure (Daunt et al., 2017). There was no evidence for adverse effects from the fishery on guillemot, puffin or razorbill (Searle et al., 2022).
- Whilst other seabirds do feed on sandeel, kittiwakes may be particularly susceptible to changes in sandeel abundance as they are surface feeders. Other seabirds are able to access more of the water column and therefore a greater proportion of sandeel so continue to survive and breed despite decreases in absolute abundance (Daunt et al., 2017).
- Fishing can also affect seabirds by changing the prey-predator balance of different species. For example, fishing mortality of sprat has an adverse impact on whiting, which reduces predation pressure on whiting prey species, such as sandeel. Fishing of herring has an adverse effect on haddock, but positive effects on Norway pout, sprat and the copepod Calanus finnmarchicus, leading to increased saith and whiting but decreases in the copepod C. helgolangicus which could benefit sandeel ( Figure 4.1 Open ▸ ; Lynam et al., 2017). The complexity of these interactions demonstrates the complexity of marine food webs.
6.3. Offshore wind energy
6.3. Offshore wind energy
- Expansion of renewable wind energy generation in marine environments may have population, community and ecosystem-level indirect impacts by introducing new habitat, locally restricting fishing and altering physical processes (van der Molen et al., 2014; Cazenave et al., 2016; Zijl et al., 2021).
- The submerged parts of offshore wind farms, such as the foundations and any scour or cable protection on the seabed, introduces new hard substrate. Typically, offshore wind farms are placed in soft substrate environments, so addition of hard substrate results in a change of habitat type which acts like an artificial reef. In the North Sea, natural reefs are rare, so whilst sandy habitat is lost, there is an increase in available habitat for reef formation (Coolen, 2017). Artificial habitats have been reported to have a positive effect on the production of benthic carnivores, due to the increased food availability and the low fishing mortality (Cresson et al., 2019).
- Artificial reefs are populated in a variety of ways. Mobile organisms can walk or swim to the new reef, animals may travel on floating objects, or pelagic larvae may be transported to the reef through currents. Artificial reefs can also act as stepping-stones, which allow organisms to colonise areas not typical of their species or they may increase the connectivity between natural sub-populations (Coolen et al., 2017). The impacts of this can extend beyond the scale of a single operation (e.g. at the scale of individual wind turbines or Project scale) with multiple adjacent offshore wind farms creating stepping stones over wider areas and creating a large-scale effect (Degraer et al., 2020). For example, the Project is adjacent to several offshore wind farms in the Forth and Tay area: Seagreen 1 and Seagreen 1A Project to the north, Inch Cape to the northwest and Neart na Gaoithe to the west.
- Like in communities found on natural hard substrates, vertical zonation is observed on wind turbine foundations, with different species colonising different zones ( Figure 6.1 Open ▸ ; Degraer et al., 2020). However, due to structural differences between natural reefs and wind turbine foundations, the species composition of artificial reefs can be significantly different to natural ones. For example, a monopile at the water surface may harbour species which are typical of the intertidal coastal zone, which is not natural in the offshore North Sea. Only 19 of 123 species observed at the seabed (at the base of wind turbine installations, and on scour protection) were also found in natural reefs in the North Sea (Coolen, 2017). The amphipod Jassa herdmani and crab Pisidia longicornis are rare on natural habitats (Zintzen, 2007), so whilst artificial reefs may increase local biomass and biodiversity, these communities cannot necessarily be compared to natural ones. In the North Sea, blue mussels Mytilus edulis have been observed to populate anthropogenic structures, such as oil and gas platforms (Schutter et al., 2019) and wind turbines monopiles (De Mesel et al., 2015; Maar et al., 2009). It has been predicted that should all offshore wind farms which are currently under construction, planned or consented be completed, the overall abundance of blue mussels could increase in the southern North Sea by more than 40%. This could lead to a decrease in available primary production, increased prey for species which feed on mussels, and decreased prey availability for other species due to competition between mussels and other prey species. This could lead to changes in food webs and ecosystem functioning (Slavik et al., 2019).
Figure 6.1 Example of the population structure and zonation on wind turbine foundations (Illustrated by Hendrik Gheerardyn; from Degraer et al., 2020).
- Artificial reef habitats can accommodate high densities of fish, compared to both sand-bottom habitats and natural reefs (Reubens, Degraer and Vincx, 2014; De Mesel et al., 2015; Mavari, Degraer and Vanaverbeke, 2021). This is likely due to a combination of attraction to the offshore wind farm from surrounding areas, as well as increasing the carrying capacity due to increased productivity. The increased structural complexity of the artificial reefs offers protection against predators and strong currents, and increased food availability. Species which have been demonstrated to have increased abundance around offshore wind farms in the North Sea include pouting, juvenile cod species, which feed on J. herdmani and sculpin feeding on decapods (Reubens, Degraer and Vincx, 2014; Mavari, Degraer and Vanaverbeke, 2021). Both prey species are strongly associated with subtidal artificial hard substrates such as offshore wind farms and shipwrecks (De Mesel et al., 2015). This, in turn, may attract predators and scavengers, which might include marine mammals (e.g. harbour seal harbour) seal Phoca vitulina, grey seal Halichoerus grypus, harbour porpoise Phocoena phocoena), predatory fish (e.g. saithe Pollachius virens, cod Gadus morhua, Allis shad, Alosa alosa, twaite shad Alosa fallax ) and elasmobranchs (e.g. spotted ray Raja montagui, spurdog Squalus acanthias, tope Galeorhinus galeus common skate Dipturus batis, and thornback ray Raja ecommi) in the vicinity of the Proposed Development (see volume 2, chapter 9).
- The Forth and Tay region has a variety of benthic habitats, ranging from moderately exposed rocky habitats to large areas of sand and mud, and glacial sand and soft sediments in the sheltered Montrose Basin, the Firths of Tay and Forth and the Eden Estuary (Marine Scotland, 2020). Organisms from rocky habitats close to the Proposed Development could populate the wind farm structures, creating an artificial hard-substrate environment. It is expected that the foundations and scour and cable protection will be colonised by species already occurring in the benthic subtidal and intertidal ecology study area (e.g. tunicates, bryozoa sp., mussels and barnacles which are typical of temperate seas; see volume 2, chapter 9). There are also potential beneficial effects for crab and lobster which are commercially exploited in the vicinity of the Proposed Development due to expansion of their natural habitats (Linley et al., 2007) and the creation of additional refuge areas. Post-construction monitoring surveys at the Horns Rev offshore wind farm, in Danish Waters in the North Sea, noted that the hard substrates were used as a hatchery or nursery grounds for several species, and was particularly successful for brown crab (BioConsult, 2006). As both fish and shellfish ecology study area, there is potential for benefits to the fisheries, depending on the materials used in construction of the offshore wind farm.
- Based on the outcomes of similar structures in the North Sea, whilst there is a reduction in soft-substrate habitats within the footprint of the wind farm, this could lead to an increase in productivity and fish at the wind farm resulting from the artificial reef effect (Reubens, Degraer and Vincx, 2014; De Mesel et al., 2015; Mavari, Degraer and Vanaverbeke, 2021). Overall, colonisation of foundations, scour protection and cable protection at the Proposed Development is expected to have a negligible to minor effect on marine species and diadromous species (see volume 2, chapter 9).
- Zijl et al (2021) modelled the impact of offshore wind farms on a variety of physical processes across the central and southern North Sea for two scenarios: the present wind farms (as of 2020) and future hypothetical wind farms (based on targets for offshore wind for 2050). In the Forth and Tay region, there were no existing wind farms at the time of study, and several wind farms were included in the future hypothetical scenario which are in planning, have been consented or are now under construction: Inch Cape, Neart na Gaoithe, Seagreen 1 and Berwick Bank (the Project). Although the model uncertainty was high, tentative results suggested there was no significant impact on the annual mean temperature and salinity. However, enhanced mixing could lead to a reduction in stratification in and around offshore wind farms by up to 60% in worst affected areas (equivalent to a decrease in mean vertical temperature difference of 0.5°C and 0.5 psu in 2050). This was consistent with modelling by Carpenter et al (2016), which found that offshore wind farms could have large-scale impacts by reducing wave action and stratification. This has the potential to increase food and oxygen transportation to the seabed, which may benefit benthic communities and their predators but be detrimental to water column and surface species.
- In shallow, shelf seas such as the North Sea, density stratification is one of the key physical features affecting the structure of the marine environment (van Leeuwen et al., 2015). Typically, in these areas changes to water density due to salinity and temperature become more important than other factors such as turbulence or mixing due to winds and tides (van Leeuwen et al., 2015).
- The presence of major estuaries within the wider Forth and Tay have a considerable impact on salinity and turbidity (Marine Assessment, 2020). Stratification models for this region of the North Sea characterised the area as a ‘region of freshwater influence’ where a fluctuating environment of stratification and break up is expected, as a result of riverine inflow (van Leeuwen et al., 2015). Although the area tends to already experience large fluctuations in stratification compared to the wider North Sea, the addition of offshore wind farms has the potential to affect stratification, the timing and strength of which affects primary production and therefore the distribution and abundance of higher trophic level species, such as seabirds and their prey (Scott et al., 2006; Cox et al., 2018). In relatively shallow regions, such as the Forth and Tay, the presence of wind turbines can increase water column mixing as well as the potential to reduce surface mixing by wind as less energy will be available after energy extraction (van Berkel et al., 2020). However, the Proposed Development is anticipated to have only a negligible to minor effect on physical processes such as tidal currents, wave climate and sediment transport both at project scale, and cumulatively with other nearby projects (see volume 2, chapter 7).
- Suspended sediment was predicted to increase by up to 5% inside offshore wind farms and decrease by up to 5% outside offshore wind farms at the sea surface (Zijl et al., 2021). At the seabed, a decrease of up to 5 - 10% both inside and outside offshore wind farms was predicted in some areas. The effects of the 2050 scenario on suspended sediment is in the same order of magnitude as some large-scale interventions, such as large infrastructure developments, sand mining and the release of dredged material. Increases in suspended sediment could impact the foraging success of visual hunters, such as cormorant and shags. Changes in wind and water flows also increase available nutrients at the surface and could lead to an increase in average primary production in offshore wind farm areas, particularly during the spring phytoplankton blooms. However, increased suspended sediment in offshore wind farm areas could obstruct sunlight and reduce primary production, particularly in the pelagic zone. These complex, conflicting impacts could result in highly localised net increase or decrease of suspended sediment, depending on the local conditions, making it difficult to predict the outcome at small scales.
- Approximately 48% of the Forth and Tay region is estimated to be highly disturbed from demersal fishing (Marine Scotland, 2020). This can increase the local suspended sediment, especially in environments with small particulates such as silt (O’Neill and Ivanoić, 2016). The area is also subject to extraction dredging, which can also increase suspended sediment, and has a large volume of river inputs which can influence turbidity (Marine Scotland, 2020). Whilst offshore wind farms may increase suspended sediments locally, this system naturally varies in turbidity due to its freshwater inputs, and other pressures such as fishing, and dredging also impact local suspended sediment levels (Marine Scotland, 2020). Additionally, the proposed array area is predominantly composed of slightly gravelly sand to gravelly sand, which has a low proportion of silt, with project-specific modelling showing only a localised, short-term increase in turbidity which, following the completion of the works, will return to baseline within a couple of tidal cycles. The Proposed Development is anticipated to have only a negligible to minor effect on the suspended sediment content at project scale, and cumulatively with other nearby projects at all phases of the development (see volume 2, chapter 7).
- Zijl et al (2021) also predicted that currents could be reduced by up to 0.2m/s within offshore wind farms and increase or decrease in magnitude outside of offshore wind farms by up to 0.005m/s in the 2020 scenario, and 0.2 m/s in the 2050 scenario. Drag introduced by piles could decrease the tidal amplitude (the elevation of tidal high water above mean sea level). However, it is not clear how this could impact the marine food web.
- Changes to physical processes resulting from offshore wind farm development and the addition of new hard substrates to the environment is predicted to impact benthic and pelagic communities differently. Telsnig Duffill et al (2018) used carbon and sulphur isotope analysis to determine the relative contribution of pelagic and benthic pathways to fish consumer production, including seabird prey species such as herring and mackerel, in the North Sea. Approximately 70% of fish biomass was linked to pelagic pathways, with only 30% linked to benthic systems. Mackerel and herring had the highest proportion of biomass from pelagic pathways of the 13 species studied. Increased transport of oxygen and nutrients to the seabed is likely to benefit benthic communities proportionally more than pelagic grazers such as zooplankton (Zijl et al., 2021). This could alter the competitive outcome of pelagic and benthic species, which could be detrimental to fish species that derive the majority of their biomass from pelagic sources like mackerel and herring. This could change the relative importance of various carbon and nutrient pathways through the marine food web and result in a fundamental altering of the functioning of the system.
- Ongoing research into the impact of offshore wind farms on the environment includes the “Predators + Prey Around Renewable Energy Developments” (PrePARED) project. This Crown Estate Scotland and Offshore Wind Evidence and Change Programme (OWEC) funded project aims to facilitate rapid large-scale offshore wind deployment in the UK by improving understanding of cumulative environmental impacts and benefits from offshore wind farms on protected marine mammal and seabird populations. New evidence is being gathered to look at the relationship between predators and prey distribution and behaviour, in the context of offshore wind farms. The Firth of Forth is one of two case study areas, and studies here are focussing on the fish (predator and prey) landscape, including trophic interactions and reef effects, with an aim of using these data to inform existing assessment tools (e.g. SeabORD). Fish surveys (acoustic, trawl, cameras, baited traps and tagging) and seabird surveys (GPS tracking) are planned for Quarter 3 in 2022 - 2024 in the Firth of Forth. The project will run until early 2026. Also through OWEC, the “Ecological Consequences of Offshore Wind” (ECOWind) Programme awarded two further research projects in the Firth of Forth which will commence in in autumn 2022. The ECOWind programme aims to address two challenges: (1) how will offshore wind expansion, combined with other anthropogenic pressures, affect species interactions and marine ecosystems?; and (2) how can understanding these consequences enable robust approaches to marine environmental restoration and net environmental gain?
6.4. Invasive non-native species
6.4. Invasive non-native species
- In the marine environment in Scotland, INNS include wireweed Sargassum muticum, green sea-fingers Codium fragile subsp. Tomentosoides, common cordgrass Spartina anglica, red alga Heterosiphonia japonica, acorn barnacle Austrominius modestus, Japanese skeleton shrimp Caprella mutica and leathery sea squirt Styela clava (NatureScot, 2022b). These can be introduced through a range of pathways, including through ballast water and attachment to ships and underwater equipment (NatureScot, 2022b), which has the potential to occur during offshore wind farm construction, operation and decommission. These species can outcompete native wildlife for space and food resources (NNSS, 2022). There is potential for INNS to compete with forage fish species and their prey species, which can consequently have wider implications on the food web, including on seabirds.
- The introduction of offshore wind turbines into the marine environment also provides new opportunities for species previously unable to colonise due to habitat or prey constraints. The addition of hard substrate into areas previously lacking this habitat type can cause reef effects, which can lead to localised changes to marine environments (Petersen and Malm, 2006). Some species, such as green sea-fingers, can rapidly colonise disturbed habitats, increasing the importance of INNS control during coastal and offshore development, such as in offshore wind farm construction (NNSS, 2022). Climate change induced movement of species into new areas (such as those associated with movement of prey species and warmer SSTs) is likely to further increase the potential for invasive non-native species to inhabit offshore areas, such as in the North Sea.
- Wind turbines may pose a relatively high risk of supporting INNS compared to other artificial structures, since they must be cleaned in situ and will be present in the environment for a relatively long time. Vessel traffic within offshore wind farms may also contribute significantly to the risk of INNS being introduced to an area, particularly if these vessels have come from elsewhere but with similar environmental conditions. Despite the potential, no INNS have been identified during post-construction monitoring at Beatrice Offshore Wind Farm in the Moray Firth (see Appendix 22, Annex 22.8). Shown to be effective at the Beatrice Offshore Wind Farm, these risks will be mitigated at the Project through the implementation of the INNS Management Plans. Considering the number of consented offshore wind farms in the vicinity of the Proposed Development, the risk of INNS may be higher than in other areas. In addition to this, relatively weak tidal currents in the area may increase the potential for colonisation by some species as larvae are able to settle out of the water column more easily. During site-specific surveys of the Proposed Development, no INNS were observed (see Appendix 22, Annex 22.8).
- The potential for INNS to out-compete native species and reduce diversity could affect overall resilience and decrease ecosystem functioning (Roberts et al., 2017). The movement of intertidal species into offshore areas has up until recently, been relatively uncommon, generally only occurring around oil and gas installations, the number of which present in the marine environment has historically been far lower than what will be available upon the construction of the many proposed North Sea offshore wind farms likely to be developed over the next few decades (Kerckhof et al., 2011). Increased incidence of lower-trophic level intertidal and subtidal species on and around offshore wind turbines has the potential to affect which species comprise fish assemblages which will in turn, affect the seabird species which can utilise the area during foraging. Around some offshore wind farms fish species richness has been known to increase, in addition to changes to the dominant pelagic fish species (Ybema et al., 2009).
6.5. Mass mortality events
6.5. Mass mortality events
- Mass mortality events (wrecks) are generally due to severe climatic conditions such as storms (particularly during winter) and results in unexpectedly high numbers of dead or dying seabirds washed up on beaches (Birkhead, 2014; Fort et al., 2015). Prolonged and harsh storms can affect birds’ foraging efficiency and therefore, energy balance, resulting in poor body conditions and potential death (Fort et al., 2015; Mitchell et al., 2020). Wrecks have been documented for years and more frequently across the world (Anker-Nilssen et al., 2016; Camphuysen, 2019; Pashby and Cudworth, 1969; Shepard, 2021). They were found to affect multiple groups of seabirds, such as alcids, shearwaters or petrels, mainly due to their smaller energy reserves (Fort et al., 2015; Glencross et al., 2021), especially along European coasts (Anker-Nilssen et al., 2017; Camphuysen, 2019).
- The most recent wreck occurred between August and November 2021, when large numbers of auks, particularly guillemots, washed up on coasts across the North Atlantic, including coastlines in the vicinity of the Proposed Development. Autopsies were conducted on 179 carcasses washed up on the UK to determine their age, gender and body condition. All analysed birds were critically emaciated and weighed half their expected body weight, most were young, and no signs of disease such as HPAI were found. As has been documented for previous wrecks, the primary cause of mortality was determined to be starvation (Fullick et al., 2022). As British colonies primarily overwinter in the North Sea, it is thought that the majority of these birds have originated from British colonies. This is supported by the recovery of three ringed guillemot from the east coast of Scotland in the late summer of 2021 (SEAPOP, 2022).
- Studies including post-mortems have been conducted to understand the potential additional causes of such wrecks, and although oil, plastic, mercury pollution and exhaustion were found, none were considered to be significant enough to cause the death of thousands of birds. Most studies attribute the wrecks to a combination of starvation and exhaustion, relating to poor weather conditions and reduced prey availability (Anker-Nilssen et al., 2017; Camphuysen et al., Heubeck et al., 2011; Morley et al., 2016). Increased extreme weather resulting from climate change could affect prey availability, weakening seabirds before strong winter storms (Camphuysen et al., 1999; 2019; Glencross et al.,2021), thus contributing to the frequency of wrecks occurring.
7. Role of offshore wind in tackling climate and biodiversity emergencies
7. Role of offshore wind in tackling climate and biodiversity emergencies
- Offshore wind has the potential to reduce climate change impacts by providing an alternative source of energy generation with lower emissions than traditional methods, which can be used alongside other renewable energy technologies such as solar (Cranmer and Baker, 2020). The addition of offshore wind farms in the marine environment provides solutions towards reaching long and short-term international energy goals (Diaz and Guedes Soares, 2020).
- In 2020, Europe had a total installed offshore wind capacity of 25GW (WindEurope, 2021). As of the end of 2020, the UK had the highest cumulative capacity of grid-connected offshore wind power in Europe (WindEurope, 2021). This is set to rise with the addition of Round 4 and ScotWind leasing rounds and demonstrates the importance of offshore wind in the UK’s plan to meet net biodiversity gain targets (BEIS, 2020). Investing in offshore wind to provide alternative energy production is desirable, due to the reduced carbon footprint, lower visual impact compared to onshore sites, the relatively high mean wind speed and the higher associated capacity factors (Green and Vasilakos, 2011; Kaldellis and Apotolou, 2017; ORE Catapult, 2021).
- Currently, positive effects of offshore wind farms are not readily considered outside of the comparison of carbon outputs compared to fossil fuel energy generation. There is potential for further positive effects from offshore wind farms, such as those associated with artificial reef habitats and the rapid implementation of compensatory measures towards a net-gain of biodiversity (RSPB, 2022). Comparison of the potential impacts on marine fauna attributed to climate change with immediate effects from energy generation in the marine environment would be beneficial.). There is a need to understand whole ecosystem responses of climate change and large-scale renewable developments, such as offshore wind, especially given the growth of offshore wind and increasing cumulative impacts. Further research is needed to predict, quantify, and separate possible ecological costs of changes due to climate change from those of large-scale renewable developments (Sadykova et al. 2018).
- Predictions of ecosystem response to offshore wind and other anthropogenic drivers are limited by the lack of understanding of the drivers of ecosystem functioning, and how they interact collectively at different trophic levels. The majority of research focuses on the relationship between apex predators or commercially important species and their prey, or behaviour of protected species individually, without considering how they interact with other organisms of the same trophic level, or those in other trophic levels. Future research on predator-prey interactions, including PrePARED will improve the understanding of cumulative environmental impacts and benefits from offshore wind farms. The ECOWind project will also address knowledge gaps in how offshore wind expansion interacts with other anthropogenic pressures and affects the marine ecosystem which will be key to inform environmental restoration.
8. Future for seabirds: predicted climate change impacts
8. Future for seabirds: predicted climate change impacts
- Under predicted climatic conditions, global ocean temperatures are expected to rise, with studies in the North Sea predicting an increase of approximately 3⁰C by 2100 (Schrum et al., 2016), in addition to other changes such as increased acidification and frequency of extreme weather events (Mitchell et al., 2020). Projections indicate rising ocean temperatures may alter marine food webs, including the quality, availability, and diversity of prey available to seabird populations (Mitchell et al., 2018) as previously discussed in section 6.1. The availability of phytoplankton to higher trophic level species supporting seabirds may be reduced with increased ocean acidification (Riebesell et al., 2013, Richier et al. 2014), possibly compounded by increased incidence of species targeting phytoplankton such as jellyfish, caused by increased ocean acidity, eutrophication and overfishing (Brotz et al., 2012; Báez et al., 2022).
- Many seabird species occupy thermal niches, with preference for warmer or colder waters affecting the ability for species to cope with changes to ambient conditions. Seabird species favouring cooler water, such as puffin, kittiwake, great black-backed gull and herring gull are predicted to have an adverse response to climate induced increased water temperature (Searle et al., 2022), with a projected 67.9% decline in the abundance of puffins in the UK estimated (Johnston et al., 2013). Forecasting models predict that populations and distributions of seabird species such as kittiwake and auks, could be greatly reduced as a result of increasing sea temperature around the UK (Mitchell et al., 2020; Sadykova et al., 2020).
- Climate change may cause large scale shifts in seabird distribution. In the North Sea, a northwards shift has been predicted for guillemot and razorbill, in addition to more substantial differences between summer and winter densities (Searle et al., 2022). Changes to the European range of UK seabirds are predicted to occur, with a decline in range expected for 65% of UK breeding seabird species (Russell et al., 2015). For guillemot, the future distribution in Europe is projected to overlap only 54-62% with the current distribution, and only 41% for razorbill (Huntley et al., 2007; Russel et al., 2015). Distributional changes linked to climate change predicted that several species such as Leach’s storm petrel Oceanodroma leucorhoa and some skua species could be extinct as breeding species in the UK by 2100 (Huntley et al., 2007; Russell et al., 2015). Huntley et al. (2007) also predicted that the Forth and Tay region will become climatically unsuitable for kittiwake, puffin and razorbill by 2100. Many seabird species are faithful to their breeding colonies potentially constraining their capacity to redistribute or expand their range in response to climate change (Mitchell et al., 2020).
- Sadykova et al (2020) predicted the spatial trends of predators and prey species around the UK in 2050 under climate change scenarios. This study used Bayesian hierarchal joint models with integrated nested Laplace approximation (INLA) to predict future spatial density distributions of predators, including guillemot, kittiwake and gannet, and the prey species sandeel and herring. Gannet and guillemot were overall projected to increase in overlap with sandeels. Kittiwake were predicted to decrease in their spatial overlap with sandeel and herring which are key prey for the species. However, these models must be interpreted with caution, as they will more accurately reflect losses than they will gains, since the future projections are not constrained by the presence (or lack) of suitable breeding sites and sediment type for prey. They also do not consider the potential for increased inter- and intra- specific competition resulting from these shifts in spatial distribution.
- As discussed in section 6.1.2, predicted northward shifts of lower trophic level species with climate change are likely to underpin changes in distribution of many seabird species in the North Sea (Lynam et al., 2017; McGinty et al., 2021). SST and salinity affect phytoplankton and zooplankton productivity, which can cascade along the food web through trophic mismatch and changes to prey availability due to mixing of the water column (Force et al., 2015; McGinty et al., 2021), possibly leading to less efficient energy and carbon transfer up to higher trophic levels (Lewandowska and Sommer, 2010). For some seabird species, such as kittiwake, spatial overlap with prey species is predicted to decrease by around 50% by 2050 (Sadykova et al., 2020).
- The European range of herring gull and lesser black-backed gull is projected to be reduced and to shift northwards, with their southern-most extent becoming climatically unsuitable (Huntley et al., 2007; Russel et al. 2015). Consequently, within the UK, gull populations have been generally predicted in to increase in response to warming climates in the UK, primarily related to their availability of prey (Johnston et al., 2021).
- As discussed in section 6.1, the breeding success of many seabird species has been linked to primary consumer availability (Carroll et al., 2015), with the location of many seabird colonies in the North Sea correlated with areas of high zooplankton abundance (Fredriksen et al., 2013). Weaker, later stratification and lower SST has been associated with higher breeding success in kittiwake colonies across the UK, including at the Isle of May and St Abbs Head NNR (some of the closest monitored populations to the Proposed Development) (Carroll et al., 2015). Under climate change, stronger and earlier stratification is predicted, which contributes to a predicted 37.3% and 32.2% decline in breeding success for kittiwake at the Isle of May and St Abbs Head NNR, respectively, by 2070-2099 (Carroll et al., 2015). Puffin and kittiwake are expected to experience declines in productivity to the projection period of 2070-2099 and are unlikely to be able to increase their foraging range to compensate (Searle et al., 2022). The same is true for great black-backed gull across the same period. Searle et al (2022) predicted a decrease in population size, whereas Sadykova et al (2020) had greater uncertainty in their predictions, from -13.0 to +4.6% by 2050. Gannet productivity is expected to increase (Searle et al., 2022), which may be attributed to their flexibility in prey species and foraging strategy and the large predicted spatial overlap with potential prey species (Davies, 2012; Sadykova et al., 2020).
- Comparing the adverse effects of climate change and the presence of offshore wind farms on seabirds is challenging, due to the dynamic, complex nature of marine ecosystems (Scott, 2022). Whilst there is potential for adverse effects from offshore wind farms, adverse ecological effects relating to climate change are predicted to increase but may be reduced by renewable energy and have an overall positive effect on ecosystem health (Figure 8.1 ;Scott, 2022). In order to ensure the future of seabirds, a holistic, ecosystem-based approach is required, with a suite of restoration measures to address habitat modifications, disruption to food webs, remove invasive species and HPAI (RSPB, 2022). Reducing the pace and magnitude of climate change effects is an important factor in a holistic approach, with offshore wind development considered a key part of the solution (European Commission, 2020; ORE Catapult 2021; RSPB, 2022; Scottish Government, 2021).
Figure 8.1. The ecological effects of climate change (represented in red) are increasing rapidly with time but is reduced by renewable energy. The comparative magnitude of effects from renewable industry in today’s climate is indicated in green (Scott, 2022).
- As presented in this technical report, there is extensive evidence that climate change both directly and indirectly is having wide scale impacts on seabirds across the North Sea. Few studies have attempted to rank the significance of pressures on seabirds. Dias et al (2019) concluded that 20.5% of global seabirds are exposed to climate change impacts, compared with just 2.2% for energy production and mining, of which offshore wind is only one component. In the Forth and Tay region, auks and kittiwake are predicted to be particularly vulnerable to the impacts of climate change, both independently and in combination with other anthropogenic pressures ( Table 8.1 Open ▸ ; Burthe et al., 2014).
8.1. Assessment of anthropogenic pressures on key seabird species
8.1. Assessment of anthropogenic pressures on key seabird species
- The impacts on seabird populations due to changes such as climatic and prey availability (discussed in sections 5.1 and 6.1), outbreaks of HPAI (section 5.4) and mass mortality events such as experienced for auks in winter 2021/22 (section 6.1) could increase in severity and frequency. The development of offshore wind farms, such as the Project, is anticipated to make a significant positive contribution to reducing greenhouse gas emissions, contributing to reducing climate change impacts (section 7; Figure 8.1 ;Scott, 2022). Possible increases in prey availability around offshore wind farms may lead to decreased commuting and foraging time for seabirds, in addition to decreased stratification of the water column in the immediate vicinity of wind turbines, the opposite of which is predicted under current climatic predictions (De Deominicis et al., 2018; Scott, 2022).
- The vulnerability of seabird communities in the Forth and Tay region to offshore wind development and climate change was considered by Burthe et al. (2014), together with a range of other threats including the indirect effects of commercial fisheries, disturbance, and pollution. Colony and at-sea seabird demographic data for 1980 – 2011 and SST data were analysed to understand seabird species vulnerability to climate change and to generate an index of population concern. Vulnerability to other threats was assessed qualitatively. The results of this study for the key seabird species present within the Proposed Development are summarised in Table 8.1 Open ▸ . The information presented in Burthe et al (2014) is supported by the literature review of the impacts of threats on seabirds in the previous sections of this report.
Table 8.1: A summary of the vulnerability of key seabird species to anthropogenic threats, their population status and the index of concern which takes into account the magnitude of vulnerability to the threats, the number of threats they are vulnerable to, their population status and their conservation status (Burthe et al., 2014).
8.1.1. Gannet
- Burthe et al. (2014) concluded that gannets were less vulnerable to climate effects, than the effects from fisheries and offshore wind farms. Gannets are predicted to be buffered from climate change effects due to their wide range of prey types, ability to forage long distances from the colony, and the ability to dive into the water column and so have access to prey at wider depths (Hamer et al., 2001; Johnston et al., 2021). Gannet at Bass Rock, near the Proposed Development, predominantly feed on sandeel and mackerel (Lewis et al, 2003), both of which are thought to be in decline in the North Sea, potentially due to climate change (Burrows et al., 2019; Clausen et al., 2017; Jansen et al., 2012; MacDonald et al., 2019). The ability of gannets to consume diverse prey types may allow them to exploit other species which currently occur in the area, or warm-water species such as horse mackerel which may increase in dominance with climate change (Burrows et al., 2019). They may also be able to take advantage of strong winds during adverse weather, making them more resilient to storms (Lane et al., 2019). This is consistent with Burthe et al. (2014), who concluded that gannet in the Forth and Tay area are highly resilient to climate change ( Table 8.1 Open ▸ ). Despite their vulnerability to displacement from offshore wind and fisheries, overall gannets were also predicted to be of low population concern to anthropogenic threats (Burthe et al., 2014). Additionally, there were no gannet bycatch offshore of the east cost of mainland Scotland, suggesting this is not a common threat to gannets specifically in the Proposed Development (Northridge, Kingston and Coram, 2020).
8.1.2. Guillemot
- Burthe et al. (2014) concluded that guillemots were vulnerable to both climate effects, and anthropogenic threats; particularly disturbance. Votier et al., (2005) estimated a decline in guillemot populations with climate change from a weak association between guillemot and forage fish abundance and SST change, and from increasing stormy weather events at Skomer Island, Wales, and so may not be representative of the response of guillemot in the vicinity of the Proposed Development. Lahoz-Monfort et al. (2011) concluded that previous guillemot abundances could be partially explained by climate indicators (the North Atlantic Oscillation and SST).
- Guillemot population abundance have been closely linked with the success of their prey species in a number of studies, both in the North Sea (e.g. Rindorf, Wanless and Harris, 2000; Frederiksen et al., 2006; Burthe et al., 2012) and further afield (e.g. Barrett and Erikstad, 2013; Erikstad et al., 2013; Lauria et al., 2012). Therefore, it is likely that the response of guillemot to indirect climate change effects, such as increasing SST, will be underpinned by their diet and the success of their prey (Johnston et al., 2021). As pursuit divers, guillemot are able to forage at a range of depths, which may make them more resilient to changes in prey availability and to climate change effects (Furness and Tasker, 2000; Johnston et al., 2021). In approximately the last 20 years, a shift from sandeel to sprat and herring has been observed in breeding guillemot at the Isle of May, which is located in the Firth of Forth (Wanless et al., 2018). This may be evidence of the ability of guillemot in the Firth of Forth area to adjust their target prey species in response to changing prey availability. However, guillemot are also vulnerable to storm events and are frequently are seen as part of wrecks (see Section 6.4 Mass Mortality Events). The combined impact of reduced prey availability from climate change and increased frequency of storms could lead to an increase of mortalities in guillemot, and an increase in wrecks featuring this species (Camphuysen et al., 2019; Harris and Wanless, 1996; Newell et al., 2015). Guillemots are also vulnerable to anthropogenic threats, including oil spills and fisheries bycatch ( Table 8.1 Open ▸ ; Burthe et al., 2014; Votier et al., 2005), which may be more significant pressures on abundances than those relating to climate change (Johnston et al., 2021).
8.1.3. Herring gull and lesser black-backed gull
- Burthe et al., (2014) concluded that herring gull and lesser black-backed gull are less vulnerable to climate change than other threats. These gull species have been considered together here, due to the similarity of how they are affected by climate and anthropogenic pressures.
- Whilst herring gull have been in decline and lesser black-backed gull populations have been unstable, it is predicted they will increase in Scotland in response to climate change pressures, because of their adaptability and generalist feeding nature (Burthe et al., 2014; Johnston et al., 2013; Johnston et al., 2021). However, management of fisheries discards and landfill, which have previously been important food sources for gulls, in the Forth and Tay region may be further detrimental to the foraging success of these species moving forward. Declines are also likely attributed to culling events, with 150,000 herring gull and lesser black-backed gull estimated to have been killed between 1970 and 1985, including 41,000 herring gulls on the Isle of May (Coulston, 2015). In Scotland, a licence has been required since 2010; however, there is limited information publicly available about licenced culls. However, an increase in lesser black-backed gulls in the 1990s on the Isle of May has been attributed to cessation of gull control measures (JNCC, 2021e). Both species are able to use urban environments for nesting, which makes them more resilient to nest failure relating to an increase in storm and flooding events, and increased competition for suitable nesting habitats, as well as pressures from coastal urbanisation (Balmer et al., 2013; Rock and Vaughan, 2013). The estimated decline in Scotland has coincided with distributional trends away from coastal areas towards inland and urban sites in both species (Balmer et al., 2013; JNCC, 2021d), where they may be more likely to be culled. Burthe et al., (2014) concluded that both species have high sensitivity to pressures from wind farms. Given the sensitivity of herring gull and lesser black-backed gull to the availability of human food sources, fisheries discards, disease and INNS, the success of populations in the Forth and Tay region may be more significantly influenced by these pressures than climate change (Burthe et al., 2014; Johnston et al., 2021).
8.1.4. Kittiwake
- Burthe et al., (2014) concluded that kittiwake have moderate to high vulnerability to all the pressures assessed, including high vulnerability to climate change. With climate change SST predicted to increase in the Forth and Tay region and across the North Sea, which may be detrimental to kittiwake success through bottom-up impacts on their preferred prey, sandeel (JNCC, 2021f; Burthe et al., 2014; Carroll et al., 2017; Wanless et al., 2018). In the North Sea, changes to the timing of key life history events in sandeel and their copepod prey have resulted in reduced quality and quantity of prey available for kittiwake (Frederiksen et al., 2013). Kittiwake overwintering survival and breeding success have been negatively correlated with an increase in SST (Frederiksen et al., 2004, 2005, 2007) and stronger and earlier stratification, including at colonies close to the Proposed Development (Carroll et al., 2015). This could be amplified by the continued fishing of sandeel, which places additional pressure on this important stock (ICES, 2019; Searle et al., 2022). As kittiwake are exclusively surface feeders, they are limited to prey available at the surface which makes them highly sensitive to reductions in prey abundance (Burthe et al., 2014; Daunt et al., 2017). These factors, along with range shifts have contributed to predictions that kittiwakes are likely to decline in the Forth and Tay region within the next 80 years (Searle et al. 2022; Sadykova et al. 2020). A reduction in prey availability will also decrease the body condition of kittiwake, meaning they are less resilient to storm conditions, which may make mortality and wreck events more likely as storms events increase in magnitude and frequency as a result of climate change (Camphuysen, 2019; Fort et al., 2015; Mitchell et al., 2020). Storms have also been demonstrated to have a direct negative effect on breeding success, through damage to nests on cliffs at the Isle of May (Newell et al., 2015). Due to the vulnerability of kittiwake to bottom-up trophic processes related to climate change and anthropogenic pressures, kittiwake populations are predicted to experience significant declines. Therefore, efforts to lessen the impact of climate change are likely to be important to this species’ success in the future.
8.1.5. Puffin
- Burthe et al., (2014) concluded that puffin have the highest vulnerability to climate effects, and effects from human disturbance, followed by effects from fisheries. The success of puffin has been closely linked with the success of their prey, making them vulnerable to bottom-up impacts relating to climate change and anthropogenic pressures (e.g. Burthe et al., 2014; Fayet et al., 2021; Frederiksen et al., 2013, Johnston et al., 2021). In the Forth and Tay area, sandeels are the dominant food in breeding puffins (Wanless et al., 2018), making them vulnerable to reduced sandeel availability, size and quality which has been associated with increasing SST, and changes in the timing of suitable available sandeel (Burthe et al., 2012; Harris, Murray and Wanless, 1998; Johnston et al., 2021; Wanless et al., 2018). Puffins are pursuit divers, meaning they are able to forage at a range of depths and which may be advantageous with climate change compared with surface feeders (Burthe et al., 2014). However, declines in puffin populations have been predicted due to their sensitivity to sandeel abundance and SST, a reduction in the overlap of puffin and sandeel ranges in the future (Sadykova et al., 2020; Searle et al., 2022), as well as predictions that the Forth and Tay region will become climatically unsuitable for puffin by 2100 (Huntley et al., 2007). This could be amplified by the continued fishing of sandeel, which places additional pressure on this important stock (ICES, 2019), and their vulnerability to nest flooding during strong rains (Newell et al, 2013). Puffin breeding success in the Forth and Tay region may also rely on the continued control of the invasive tree mallow plant (Anderson, 2021a; Anderson, 2021b). Burthe et al. (2014) concluded that puffins have low vulnerability to pressures associated with wind farms. Puffins are not considered vulnerable to collision risk due to their flight height, high proportion of time spent loafing and underwater, and their manoeuvrability (Furness et al., 2013).
8.1.6. Razorbill
- Burthe et al., (2014) concluded that razorbill have a greater vulnerability to climate effects and disturbance, than the other pressures assessed. Whilst bottom-up influences associated with the availability of sandeel have been correlated with razorbill productivity, the biomass of 0-group (Burthe et al., 2012) and 1-group (Frederiksen et al., 2006) sandeels was not found to have a significant effect on razorbill productivity. Evidence that they can exploit other food sources such as sprat and herring (Wanless et al., 2018) may have contributed to their success compared with kittiwake, for example, which has also been correlated with sandeel abundance (Frederiksen et al., 2004, 2005, 2007). However, despite recent increases in population size near the Proposed Development, it is predicted that razorbill will be vulnerable to climate impacts due to a reduction in overlap with sandeel stocks (Burthe et al., 2014; Searle et al., 2022), and predictions that the area may become unsuitable in the future (Huntley et al., 2007). The combined impact of climate change from reduced prey availability and increased frequency of storms could lead to an increase in mortalities in guillemot, and an increase in wrecks featuring this species (Camphuysen et al., 2019; Harris and Wanless, 1996; Newell et al., 2015). Changes to prey availability may also leave razorbill increasingly vulnerable to wrecks associated with storm events, as they may not have sufficient energy stores to survive storm conditions (Heubeck et al., 2011). Storms have also been demonstrated to have a direct negative effect on breeding success, through damage to nests on cliffs at the Isle of May which could continue to be detrimental to the productivity of the species (Newell et al., 2015). Razorbills have moderate vulnerability to anthropogenic threats, including fisheries bycatch and offshore wind which may contribute to pressures on the species ( Table 8.1 Open ▸ ; Burthe et al., 2014), although razorbill are at low risk of collision with offshore wind farms due to their low flight height and high flight agility (Furness et al., 2013).
9. Summary
9. Summary
- The purpose of this Technical Appendix is to put the potential impacts from the Proposed Development on seabirds in the context of the wider ecosystem for seabird species which are relatively abundant in the area of the Proposed Development. Seabirds which are sensitive to potential impacts from the Proposed Development are: gannet, guillemot, herring gull, kittiwake, lesser black-backed gull, puffin and razorbill.
- Scotland is estimated to support 45% of Europe’s breeding seabirds, including 11% and 43% of Europe’s kittiwake and guillemot populations respectively, and approximately 46% of the world’s gannet population (Forrester et al., 2007; Murray, Harris and Wanless, 2015). The Forth and Tay region supports significant colonies of all of the key species considered in this Technical Appendix. Population declines have been observed in breeding seabirds in Scotland between 1986 – 2018 (Marine Scotland, 2020). raising concern for the future of Scotland’s seabirds. This includes declines of herring gull, kittiwake and puffin in the Forth and Tay area since the first census in 1969-70 (JNCC, 2021d JNCC, 2021f; JNCC, 2021g).
- Population declines and breeding failures have been linked with a range of direct and indirect pressures on seabirds. Direct threats to seabirds include incidental capture and entanglement in fishing gear, vessel disturbance, collision, and displacement from offshore wind farms, changes in weather due to climate change, disease and INNS (Žydelis et al. 2013; Dias et al., 2019; Fliessbach et al., 2019; Mitchell et al., 2020). As top-predators in the marine environment, seabirds are sensitive to bottom-up pressures, including effects on the lowest trophic levels (e.g. on plankton and copepods), and mid-trophic levels (e.g. prey fish species). Sandeels, followed by sprat and herring, are some of the most important prey species for Scotland’s seabird populations (Wanless et al., 2018). The most significant indirect threats to seabirds are related to the reduced availability of these prey species, such as through fishing, and food web impacts related to climate change (MacDonald et al., 2015; Lynam et al., 2017; Mitchell et al., 2020; Marine Scotland, 2020).
- However, it is challenging to separate the effects of different pressures, due to the complexity of how they interact and the combined impact they have on seabird populations, their environment and their prey at all scales. Although offshore wind farms can impact local seabird populations directly through displacement and collision, there may also be beneficial indirect impacts from offshore wind farms, for example through the creation of artificial reefs in wind turbine foundations to increase prey availability for some seabird species (Coolen, 2017).
- Overall, gannet, herring gull and lesser black-backed gull are thought to be buffered from the impacts of climate change, mostly relating to their ability to access a wider variety of prey but may be sensitive to controls on fisheries discards (Johnston et al., 2021). Guillemot, kittiwake, puffin and razorbill abundances have been more closely linked to the success of their prey, which may make them more vulnerable to bottom-up climate change impacts (Burthe et al., 2014; Johnston et al., 2021). A reduction in prey quality and availability may also reduce the resilience of these species against storm events, which could lead to an increase in large-scale wrecks as climate change leads to an increase in extreme weather (Anker-Nilssen et al., 2017; Camphuysen et al., Heubeck et al., 2011; Morley et al., 2016). Cliff nesting species, such as kittiwake and razorbill, may also be sensitive to nest failure in high winds and storm surges (Newell et al., 2015). Whilst auks and gannet may be sensitive to fisheries bycatch, high-risk fishing gear such as static net, longline and midwater trawls, are not common in the Forth and Tay region (Bradbury et al., 2017; Larsen et al., 2021). In the Forth and Tay region, and elsewhere, gannet, herring gull, kittiwake and lesser black-backed gull may also be vulnerable to effects from offshore wind farms, including collision and displacement (Burthe et al., 2014; Furness et al., 2013).
Whilst there is uncertainty around the in-combination effects from a growing number of windfarms, without action to lower carbon emission, climate change related impacts are likely to continue having an adverse effect on seabird populations, which must be considered when weighing up ecological trade-offs (Scott, 2022).
- Without intervention to reduce the pace and severity of climate change, the status of seabird populations will be adversely affected through direct and indirect impacts. Whilst climate change is arguably the biggest driver of change, other pressures including pollution, fisheries and offshore developments all contribute. In order to ensure the future of seabirds, a holistic, ecosystem-based approach is required, with a suite of restoration measures to address habitat modifications, disruption to food webs, removal of invasive species and HPAI (RSPB, 2022). Climate change is leading to ecosystem-wide environmental changes through factors such as rising global sea levels, increasing frequency of severe weather events, and warming oceans, with adverse effects on seabirds predicted to continue if action is not taken (McGinty et al., 2021; Mitchell et al., 2020; Sadykova et al., 2020; Searle et al., 2022). Offshore wind farms will play a vital role in reducing the impacts of climate change by generating electricity with low carbon emissions compared with fossil fuels (European Commission, 2020; ORE Catapult 2021; Scottish Government, 2021), and can provide an opportunity to benefit seabirds through rapid implementation of compensatory measures (RSPB, 2022).
- This technical appendix has outlined the direct and indirect impacts to seabird populations from key threats, including climate change, offshore wind farms and commercial fisheries. This has shown the complexity of marine food webs, and the pressures to seabirds and the wider marine ecosystem. Climate change is one of the most significant threats to this system. Whilst offshore wind farm development may have local negative impacts, on a wider scale offshore wind farms are anticipated to make a significant positive contribution to reducing greenhouse gas emissions, contributing to reducing climate change impacts.
10. References
10. References
Alder, J., Campbell, B., Karpouzi, V., Kaschner, K. and Pauly, D. (2008). Forage Fish: From Ecosystems to Markets. Annual Review of Environment and Resources, 33, pp.153-166.
Anderson, O.R., Small, C.J., Croxall, J.P., Dunn, E.K., Sullivan, B.J., Yates, O. and Black, A. (2011). Global seabird bycatch in longline fisheries. Endangered Species Research, 14(2), pp.91-106.
Anker-Nilssen, T., Harris, M.P., Kleven, O. and Langset, M. (2017). Status, origin and population level impacts of Atlantic puffins killed in a mass mortality event in the southwest Norway early 2016. Seabird, 30, 1-14.
Avery-Gomm, S., Valliant, M., Schacter, C.R., Robbins, K.F., Liboiron, M., Daoust, P-Y., Rios, L.M. and Jones, I.L. (2016). A study of wrecked Dovekies (Alle alle) in the western North Atlantic highlights the importance of using standardized methods to quantify plastic ingestion. Marine Pollution Bulletin, 113, 75-80.dov
Báez, J.C., Pennino, M.G., Albo-Puigserver, M., Coll, M., Giraldez, A. and Bellido, J.M. (2022). Effects of environmental conditions and jellyfish blooms on small pelagic fish and fisheries from the Western Mediterranean Sea. Estuarine, Coastal and Shelf Science, 264, pp.107.
Balmer, D. E., Gillings, S., Caffrey, B. J., Swann, R. L., Downie, I. SS. and Fuller, R. J. (2013), Bird Atlas 2007–11: the breeding and wintering birds of Britain and Ireland. BTO Books, ThetfordBand, B. (2012). Using a Collision Risk Model to Assess Bird Collision Risks for Offshore Wind Farms. Report by British Trust for Ornithology (BTO), Bureau Waardenburg bv and University of St Andrews.
Banyard, A. C., Lean, F., Robinson, C., Howie, F., Tyler, G., Nisbet, C., Seekings, J., Meyer, S., Whittard, E., Ashpitel, H.F. and Bas, M. (2022). Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses, 14(2), pp.212.
Barrett, R.T. and Erikstad, K.E. (2013). Environmental variability and fledging body mass of Common Guillemot Uria aalge chicks. Marine Biology, 160, pp.1239–1248.
BEIS (2020). The Energy White Paper: Powering our Net Zero Future. 1st ed. London. ISBN 978-1-5286-2219-6.
BioConsult (2006). Hydroacoustic Monitoring of Fish Communities at Offshore Wind Farms, Horns Rev Offshore Wind Farm, Annual Report 2005.
BirdLife International. (2018). State of the World's Birds: Taking the Pulse of the Planet. BirdLife International, Cambridge, UK.
Birkhead, T. (2014). The SeabirdWreck of 2014. Available at: https://myriadbirds.com/2014/03/03/wrecks/. Accessed on: 7 July 2022.
Blew, J., Hoffmann, M., Nehls, G. and Hennig, V. (2008). Investigation of the birds collision risk and the response of harbour porpoises in the offshore wind farms Horns Rev, North Sea and Nysted, Baltic Sea in Denmark. Part 1: Birds. Final Report.
Boulcott, P., and Wright, P.J. (2008). Critical timing for reproductive allocation in a capital breeder: evidence from sandeels. Aquatic Biology, 3(1), pp.31-40.
Bowgen, K. and Cook, A. (2018). Bird Collision Avoidance: Empirical evidence and impact assessments. JNCC Report No: 614. JNCC, Peterborough.
Bradbury, G., Shackshaft, M., Scott-Hayward, L., Rextad, E., Miller, D. and Edwards, D. (2017). Risk assessment of seabird bycatch in UK waters. Report to Defra. Defra Project: MB0126.
Brotz, L., Cheung, W.W., Kleisner, K., Pakhomov, E. and Pauly, D. (2012). Increasing jellyfish populations: trends in large marine ecosystems. Jellyfish blooms, 4, pp.3-20.
Burger, A. E. and Simpson, M. (1986). Diving depths of Atlantic puffins and common murres. The Auk, 103, pp.828-830.
Burrows, M.T., Bates, A. E., Costello, M.J., Edwards, M., Edgar, G. J., Fox, C. J., Halpern, B. S., Hiddink, J. G., Pinsky, M. L., Batt, R. D., Molinos García, J., Payne, B. L., Schoeman, D. S., Stuart-Smith, R. D. and Poloczanska, E. S. (2019). Ocean community warming responses explained by thermal affinities and temperature gradients. Nature climate change, 9, pp.959-963.
Burthe, S. J., Wanless, S., Newell, M. A., Butler, A., and Daunt, F. (2014). Assessing the vulnerability of the marine bird community in the western North Sea to climate change and other anthropogenic impacts. Marine Ecology Progress Series, 507, 277-295.
Burthe, S., Daunt, F., Butler, A., Elston, D.A., Frederiksen, M., Johns, D., Newell, M., Thackeray, S.J. and Wanless, S. (2012). Phenological trends and trophic mismatch across multiple levels of a North Sea pelagic food web. Marine Ecology Progress Series, 454, pp.119-133.
Calladine, J. R., Park, K. J., Thompson, K. and Wernham, C. V. (2006). Review of urban gulls and their management in Scotland: a report to the Scottish Executive. BTO Scotland, Stirling.
Camphuysen, C.J., Fox, A.D., Leopold M.F. and Petersen, I.K. (2004). Towards standardised seabirds at sea census techniques in connection with environmental impact assessments for offshore wind farms in the U.K. - A comparison of ship and aerial sampling methods for marine birds, and their applicability to offshore wind farm assessments. Report COWRIE - BAM -02-2002.
Camphuysen, C. J., Wright, P. J., Leopold, M., Huppop, O., and Reid, J. B. (1999). A review of the causes, and consequences at the population level, of mass mortalities of seabirds. ICES Cooperative Research Report No. 232.
Camphuysen, C.J. (2013). A historical ecology of two closely related gull species (Laridae): multiple adaptations to a manmade environment. PhD Thesis, University of Groningen.
Camphuysen, C.J. (2019). A decline in oil rates consolidated – Monitoring and assessment of the proportion of oiled common guillemots in The Netherlands – winter 2018-19. Nioz Reports, RWS Central Informatievoorziening BM 19.29.
Carey, M.J. (2011). Intergenerational transfer of plastic debris by Short-tailed Shearwaters (Ardenna tenuirostris). Emu, 111(3), pp.229-234.
Carpenter, J. R., Merckelbach, L., Callies, U., Clark, S., Gaslikova, L., and Baschek, B. (2016). Potential impacts of offshore wind farms on North Sea stratification. PLoS ONE, 11(8), e0160830.
Carroll, M.J., Butler, A., Owen, E., Ewing, S.R., Cole, T., Green, J.A., Soanes, L.M., Arnould, J.P., Newton, S.F., Baer, J. and Daunt, F. (2015). Effects of sea temperature and stratification changes on seabird breeding success. Climate Research, 66(1), pp.75-89.
Carroll, M.J., Bolton, M., Owen, E., Anderson, G.Q.A., Mackley, E.K., Dunn, E.K. and Furness, R.W. (2017). Kittiwake breeding success in the southern North Sea correlates with prior sandeel fishing mortality. Aquatic Conservation: Marine and Freshwater Ecosystems, 27(6), pp.1164-1175.
Cazenave, P. W., Torres, R., and Allen, J. I. (2016). Unstructured grid modelling of offshore wind farm impacts on seasonally stratified shelf seas. Progress in Oceanography, 145, pp.25–41.
Chimienti, M., Cornulier, T., Owen, E., Bolton, M., Davies, I.M., Travis, J.M.J. and Scott, B. E. (2017) Taking movement data to new depths: Inferring prey availability and patch profitability from seabird foraging behavior. Ecology and Evolution, 7(23), pp.10252–65.
Chistensen, T.K., Hounisen, J.P., Clausager, I. and Petersen, I.B. (2004). Visual and radar observations of birds in relation to collision risk at the Horns Rev offshore wind farm. National Environmental Research Institute. Elsam Engineering A/S.
Church, G.E., Furness, R.W., Tyler, G., Gilbert, L. and Votier, S.C. (2019). Change in the North Sea ecosystem from the 1970s to the 2010s: great skua diets reflect changing forage fish, seabirds, and fisheries. ICES Journal of Marine Science, 76(4), pp.925-937.
Clausen, L. W., Rindorf, A., van Deurs, M., Dickey-Collas, M. and Hintzen N. T. (2017). Shifts in North Sea forage fish productivity and potential fisheries yield. Journal of Applied Ecology, 55(3), pp.1092-1101.
Cook, A.S.C.P., Johnston, A., Wright, L.J. and Burton, N.H.K. (2012). Strategic Ornithological Support Services. Project SOSS-02. A review of flight heights and avoidance rates of birds in relation to offshore wind farms. BTO Research Report 618.
Cook, A.S.C.P., Dadam, D. and Robinson, R.A. (2014b). Development of MSFD Indicators, Baselines and Target for the Annual breeding Success of Kittiwakes in the UK (2012). JNCC Report No. 538, BTO for JNCC, Peterborough.
Cook, A.S.C.P., Humphreys, E.M., Masden, E.A. and Burton, N.H. (2014a). The avoidance rates of collision between birds and offshore turbines. BTO Research Report No: 656, pp.241.
Coolen, J.W.P. (2017). North Sea Reefs: Benthic biodiversity of artificial and rocky reefs in the southern North Sea. PhD Thesis. Wageningen University, Wageningen.
Cormon, X., Kempf, A., Vermand, Y., Vinther, M. and Machal, P. (2016). Emergence of a new predator in the North Sea: evaluation of potential trophic impacts focused on hake, saithe, and Norway pout. ICES Journal of Marine Science, 73(5), pp.1370-1381.
Cranmer, A. and Baker, E. (2020). The global climate value of offshore wind energy. Environmental Research Letters, 15(5), pp.54.
Cresson, P., Le Direach, L., Rouanet, E., Goberville, E., Astruch, P., Ourgaud, A. and Hamerlin-Vivien, M. (2019). Functional traits unravel temporal changes in fish biomass production on artificial reefs. Marine Environmental Research, 145, pp.137-146.
Coulston, J. C. (2015) Re-Evaluation of the Role of Landfills and Culling in the Historic Changes in the Herring Gull (Larus argentatus) Population in Great Britain. Waterbirds, 38(4), pp.339-354.
Coulson, J. C. (2019). Gulls. New Naturalist 139. Harper Collins, London.
Daunt, F. and Mitchell, I. (2013). Impacts of climate change on seabirds. MCCIP Science Review, pp.125-133.
Daunt, F., Mitchell, I. and Frederiksen, M. (2017) Seabirds. MCCIP Science Review 2017, pp.42-46.
Davies, R.D. (2012) Foraging behaviour and population dynamics of northern gannets over a period of environmental change. PhD thesis, University of Leeds.
De Dominicis, M., Wolf, J. and O'Hara Murray, R. (2018). Comparative effects of climate change and tidal stream energy extraction in a shelf sea. Journal of Geophysical Research: Oceans, 123(7), pp.5041-5067.
De Mesel, I., Kerckhof, F., Norro, A., Rumes, B. and Degraer, S. (2015). Succession and seasonal dynamics of the epifauna community on offshore wind farm foundations and their role as stepping stones for non-indigenous species. Hydrobiologia, 756(1), pp.37-50.
Degraer, S., Carey, D. A., Coolen, J. W. P., Hutchion, Z. L., Kerckhof, F., Rumes, B. and VanaVerbeke, J. (2020). Offshore wind farm artificial reefs affect ecosystem structure and functioning: A synthesis. Oceanography, 33(4), pp.48-57.
DECC. (2011). UK Renewable Energy Roadmap. Department of Energy and Climate Change, London.
DEFRA. (2022). 6 June 2022: Highly pathogenic avian influenza (HPAI) in the UK and Europe. Available at: https://www.gov.uk/government/publications/avian-influenza-bird-flu-in-europe. Accessed on: 12 August 2022.
del Hoyo, J., Elliot, A. and Sargatal, J. (eds) (1996). Handbook of the birds of the world, vol. 3. Lynx Edicions, Barcelona, Spain.
Deng, H., He, J., Feng, D., Zhao, Y., Sun, W., Yu, H., and Ge, C. (2021). Microplastics pollution in mangrove ecosystems: a critical review of current knowledge and future directions. Science of the Total Environment, 753, pp.142041.
and (2005) Avian collision risk at an offshore wind farm. Biology Letters, 1, pp.296-298.
Dias, M.P., Martin, R., Pearmain, E.J., Burfield, I.J., Small, C., Phillips, R.A., Yates, O., Lascelles, B., Borboroglu, P.G. and Croxall, J.P. (2019). Threats to seabirds: A global assessment. Biological Conservation, 237(2019), pp.525-537.
Diaz, H. and Soares, C.G. (2020). Review of the current status, technology and future trends of offshore wind farms. Ocean Engineering, 209, 107381
Dierschke, V., Furness, R.W. and Garthe, S. (2016). Seabirds and offshore wind farms in European waters: Avoidance and attraction. Biological Conservation, 202, pp.59-68.
Drewitt, A. and Langston R.H.W. (2006) Assessing the impacts of wind farms on birds. Ibis, 148, pp.29-42.
Dunn, D.C., Harrison, A.L., Curtice, C., DeLand, S., Donnelly, B., Fujioka, E.I., Heywood, E., Kot, C.Y., Poulin, S., Whitten, M. and Åkesson, S. (2019). The importance of migratory connectivity for global ocean policy. Proceedings of the Royal Society B, 286(1911), 20191472.
Durant, J. M., Anker-Nilssen, T. and Stenseth, N. C. (2003). Trophic interactions under climate fluctuations: the Atlantic puffin as an example. Proceedings. Biological sciences / The Royal Society, 270, pp.1461–1466.
Emeis, K-C., van Beusekom, J., Callies, U., Ebinghaus, R., Kannen, A., Kraus, G., Kröncke, I., Lenhart, H., Lorkowski, I., Matthias, V. and Möllmann. (2015). The North Sea—a shelf sea in the Anthropocene. Journal of Marine Systems, 141, pp.18-33.
Erikstad, K.E., Reiertsen, T.K., Barrett, R.T., Vikebø, F. and Sandvik, H. (2013). Seabird-fish interactions: The fall and rise of a common guillemot Uria aalge population. Marine Ecology Progress Series, 475, pp.267–276.
European Commission (2019). Regulation 2019/1241 on the conservation of fisheries resources and the protection of marine ecosystems through technical measures (Technical Measures Regulation) 2019. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32019R1241v
European Commission. (2020). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: An EU Strategy to harness the potential of offshore renewable energy for a climate neutral future. Brussels: European Commission. COM(2020) 741 Final.
Eurostat. (2022). Renewable energy statistics. Available at: ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics#:~:text=Share%20of%20renewable%20energy%20more%20than%20doubled%20between,2020%2C%20around%202%20percentage%20points%20above%20its%20target. Accessed on: 10 March 2022.
FAO. 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome.
Fauchald, P., Skov, H., Skern-Mauritzen, M., Johns, D. and Tveraa, T. (2011). Wasp-waist interactions in the North Sea ecosystem. PLoS ONE, 6(7), e22729.
Fayet, A.L., Clucas, G.V., Anker-Nilssen, T., Syposz, M. and Hansen, E.S. (2021). Local prey shortages drive foraging costs and breeding success in a declining seabird, the Atlantic puffin. Journal of Animal Ecology, 90(5), pp.1152-1164.
Fliessbach, K.L., Borkenhagen, K., Guse, N., Markones, N., Schemmer, P. and Garthe, S. (2019). A ship traffic disturbance vulnerability index for northwest European seabirds as a tool for Marine Spatial Planning. Frontiers in Marine Science, 6, 192.
Foster, S., Swann, R.L. and Furness, R.W. (2017). Can changes in fishery landings explain longterm population trends in gulls? Bird Study, 64, pp.90–97.
Force, M.P., Santora, J.A., Reiss, C.S. and Loeb, V.J. (2015). Seabird species assemblages reflect hydrographic and biogeographic zones within Drake Passage. Polar Biology, 38(3), pp.381-392.
Ford, H.V., Jones, N.H., Davies, A.J., Godley, B.J., Jambeck, J.R., Napper, I.E., Suckling, C.C., Williams, G.J., Woodall, L.C. and Koldewey, H.J. (2022). The fundamental links between climate change and marine plastic pollution. Science of the Total Environment, 806(1), 150392.
Forrester, R.W., Andrews, I.J., Mcinerny, C.J., Murray, R.D., Mcgowan, R.Y., Zonfrillo, B., Betts, M.W., Jardine, D.C. and Grundy, D.S. (2007). The Birds of Scotland. Scottish Ornithologists’ Club, Aberlady.
Fort, J., Lacoue-Labarthe, T., Nguyen, H.L., Boue, A., Spitz, J. and Bustamante, P. (2015). Mercury in wintering seabirds, an aggravating factor to winter wrecks? Science of the Total Environment, 527-528, 448-454.
Foster, S., Swann, R.L., and Furness, R.W. (2017). Can changes in fishery landings explain long-term population trends in gulls? Bird Study, 65(1).
Franci, C.D., Vézina, F., Grégoire, F., Rail, J.F. and Verreault, J. (2015). Nutritional stress in Northern gannets during an unprecedented low reproductive success year: Can extreme sea surface temperature event and dietary change be the cause? Comparative Biochemistry and Physiology -Part A : Molecular and Integrative Physiology, 181, pp.1–8.
Frederiksen, M., Anker‐Nilssen, T., Beaugrand, G. and Wanless, S. (2013). Climate, copepods and seabirds in the boreal Northeast Atlantic–current state and future outlook. Global change biology, 19(2), pp.364-372.
Frederiksen, M., Daunt, F., Harris, M.P. and Wanless, S. (2008b). The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird. Journal of Animal Ecology, 77, pp.1020-1029.
Frederiksen, M., Edwards, M., Mavor, R.A. and Wanless, S. (2007). Regional and annual variation in black-legged kittiwake breeding productivity is related to sea surface temperature. Marine Ecology Progress Series, 350, pp.137-143.
Frederiksen, M., Edwards, M., Richardson, A.J., Halliday, N.C. and Wanless, S. (2006). From plankton to top predators: bottom‐up control of a marine food web across four trophic levels. Journal of Animal Ecology, 75(6), pp.1259-1268.
Frederiksen, M., Jensen, H., Daunt, F., Mavor, R.A. and Wanless, S. (2008a). Differential effects of a local industrial sand lance fishery on seabird breeding performance. Ecological Applications, 18, pp.701-710.
Frederiksen, M., Wanless, S., Harris, M.P., Rothery, P. and Wilson, L.J. (2004). The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. Journal of Applied Ecology, 41, pp.1129-1139.
Frederiksen, M., Wright, P.J., Heubeck, M., Harris, M.P., Mavor, R.A. and Wanless, S. (2005). Regional patterns of kittiwake Rissa tridactyla breeding success are related to variability in sandeel recruitment. Marine Ecology Progress Series, 300, 201–211.
Fullick, E., Bidewell, C.A., Duff, J.P., Holmes, J.P, Howie, F., Robinson, C., Goodman, G., Beckmann, K. M. and Philbey, A.W. (2022). Mass mortality of seabirds in GB. Veterinary Record, 190(3), pp.129-130.
Furness, R. W. and Tasker, M. L. (2000). Seabird-fishery interactions: quantifying the sensitivity of seabirds to reductions in sandeel abundance, and identification of key areas for sensitive seabirds in the North Sea. Marine Ecology Progress Series, 202, pp.253-264.
Furness, R.W. and Camphuysen, C.J. (1997). Seabirds as monitors of the marine environment. ICES Journal of Marine Science, 54, pp.726-737.
, and (2013) Assessing vulnerability of seabird populations to offshore wind farms. Journal of Environmental Management, 119, pp.56-66.
Gibson, L., Wilman, E.N. and Laurance, W.F. (2017). How green is ‘green’energy?. Trends in ecology & evolution, 32(12), pp.922-935.
Gilbert, M. and Xiao, X. (2008). Climate change and avian influenza. Review of Scientific Technology, 27(2), pp.459-466.
Gill, A.B. (2005). Offshore renewable energy: ecological implications of generating electricity in the coastal zone. Journal of Applied Ecology, 42, pp.605-615.
Glencross, J.S., Lavers, J. and Woehler, E.J. (2021). A proposed framework for reporting mass mortality (wreck) events of seabirds. ICES Journal of Marine Science, 78(6), 1935-1942.
Good, T.P., June, J.A., Etnier, M.A. and Broadhurst, G. (2010). Derelict fishing nets in Puget Sound and the Northwest Straits: patterns and threats to marine fauna. Marine Pollution Bulletin, 60, pp.39-50.
Green, R. and Vasilakos, N. (2011). The economics of offshore wind. Energy Policy, 39(2), pp.496-502.
Greenstreet, S., Fraser, H., Armstrong, E. and Gibb, I. (2010). Monitoring the consequences of the northwestern North Sea sandeel fishery closure. Scottish Marine and Freshwater Science, 1(6), pp.1-34.
Greve, W., Reiners, F., Nast, J., and Hoffmann, S. (2004). Helgoland Roads meso- and macrozooplankton time-series 1974 to 2004: Lessons from 30 years of single spot, high frequency sampling at the only off-shore island of the North Sea. Helgoland Marine Research, 58(4), pp.274-288.
Hakkinen, H., Petrovan, S.O., Sutherland, W.J., Dias, M.P., Ameca, E.I., Oppel, S., Ramírez, I., Lawson, B., Lehikoinen, A., Bowgen, K.M. and Taylor, N.G. (2022). Linking climate change vulnerability research and evidence on conservation action effectiveness to safeguard European seabird populations. Journal of Applied Ecology, 59(5), pp.1178-1186.
Hall, R. M. (2022). The impact of avian flu. National Trust Scotland. Available at: https://www.nts.org.uk/stories/the-impact-of-avian-flu. Accessed on: 19 July 2022.
Halpern, B.S., Frazier, M., Potapenko, J., Casey, K.S., Koenig, K., Longo, C., Lowndes, J.S., Rockwood, R.C., Selig, E.R., Selkoe, K.A. and Walbridge, S. (2015). Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nature Communication, 6, 7615.
Hamer, K.C., Phillips, R.A., Hill, J.K., Wanless, S. and Wood, A.G. (2001). Contrasting foraging strategies of gannets Morus bassanus at two North Atlantic colonies: Foraging trip duration and foraging area fidelity. Marine Ecology Progress Series, 224, pp.283–290.
Harris, M.P., Albon, S.D., Newell, M.A., Gunn, C., Daunt, F., and Wanless, S. (2022). Long-term within-season changes in the diet of Common Guillemot (Uria aalge) chicks at a North Sea colony: implications for dietary monitoring. IBIS International Journal of Avian Science.
Harris, M.P., Murray, S. and Wanless, S. (1998). Long-term changes in breeding performance of puffins fratercula arctica on St Kilda. Bird Study, 45, pp.371–374.
Harris, M.P. and Wanless, S. (1996). Differential responses of Guillemot Uria aalge and Shag Phalacrocorax aristotelis to a late winter wreck. Bird Study 43, pp.220–230
Harrison, A.L., Costa, D.P., Winship, A.J., Benson, S.R., Bograd, S.J., Antolos, M., Carlisle, A.B., Dewar, H., Dutton, P.H., Jorgensen, S.J. and Kohin, S. (2018). The political biogeography of migratory marine predators. Nature Ecology & Evolution, 2(10), pp.1571-1578.
Heath, M.R., Neat, F.C., Pinnegar, J.K., Reid, D.G., Sims, D.W. and Wright, P. (2012). Review of climate change impacts on marine fish and shellfish around the UK and Ireland. Aquatic Conservation Marine and Freshwater Ecosystems, 22(3).
Heubeck, M., Aarvak, T., Isaksen, K, Johnsen, A, Petersen, I. K. and Anker-Nilssen, T. (2011). Mass mortality of adult Razorbills Alca torda in the Skagerrak and North Sea area, autumn 2007. Seabird, 24, pp.11-32.
Howells, R.J., Burthe, S.J., Green, J.A., Harris, M.P., Newell, M.A., Butler, A., Wanless, S. and Daunt, F. (2017). From days to decades: short-and long-term variation in environmental conditions affect offspring diet composition of a marine top predator. Marine Ecology Progress Series, 583, pp.227-242.
Humphreys, E. M., Cook, A. S. C. P. and Burton, N. H. K. (2015). Collision, Displacement and Barrier Effect Concept Note. BTO Research Report No: 669.
Hunter, A., Speirs, D. and Heath, M.R. (2019). Population density and temperature correlate with long-term trends in somatic growth rates and maturation schedules of herring and sprat. PLoS ONE 14(3): e0212176.
Huntley, B., Green, R.E., Collingham, Y.C. and Willis, S.G. (2007). A Climatic Atlas of European Breeding Birds. Durham University, The RSPB and Lynx Edicions, Barcelona.
ICES (2015) Report of the Joint ICES/OSPAR Working Group on Seabirds (JWGBIRD), 17–21 November 2014, Copenhagen, Denmark. ICES CM 2014/ACOM:30, 115 pp.
ICES (2017) Sandeel (Ammodytes spp.) in Division 4.a, Sandeel Area 7r (northern North Sea, Shetland). ICES Advice on Fishing Opportunities, Catch, and Effort Greater North Sea Ecoregion (san.sa.7r).
ICES (2019). ICES Standard Graphs. Available at: http://standardgraphs.ices.dk/stockList.aspx. Accessed on: 1 July 2022.
ICES. (2014). Annex 5: North Sea SMS model key run. Report of the Working Group on Multispecies Assessment Methods (WGSAM). CM 2014/SSGSUE:11, ICES.
IPCC. (2014). Summary for Policymakers. In: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.
Jansen, T. and Gislason, H. (2011) Temperature affects the timing of spawning and migration of North Sea mackerel. Continental Shelf Research, 31, pp.64–72.
Jansen, T., Kristensen, K., Payne, M., Edwards, M., Schrum, C. and Pitois, S. (2012) Long-Term Retrospective Analysis of Mackerel Spawning in the North Sea: A New Time Series and Modeling Approach to CPR Data. PLOS ONE, 7(6): e38758.
JNCC. (2021a). Seabird Monitoring Programme Report 1986 – 2019. Available at: https://jncc.gov.uk/our-work/smp-report-1986-2019/. Accessed on: 25 August 2022.
JNCC. (2021b). Northern gannet (Morus bassanus). Available at: https://jncc.gov.uk/our-work/northern-gannet-morus-bassanus/#annual-abundance-and-productivity-by-geographical-area-scotland. Accessed on: 30 August 2022.
JNCC. (2021c). Guillemot (Uria aalge). Available at: https://jncc.gov.uk/our-work/guillemot-uria-aalge/. Accessed on: 30 August 2022.
JNCC. (2021d). Herring gull (Larus argentatus). Available at: https://jncc.gov.uk/our-work/herring-gull-larus-argentatus/. Accessed on: 30 August 2022.
JNCC. (2021e). Lesser black-backed gull (Larus fuscus). Available at: https://jncc.gov.uk/our-work/lesser-black-backed-gull-larus-fuscus/v. Accessed on: 30 August 2022. v. Accessed on: 30 August 2022.
JNCC. (2021f). Black-legged kittiwake (Rissa tridactyla). Available at: https://jncc.gov.uk/our-work/black-legged-kittiwake-rissa-tridactyla/
JNCC. (2021g). Atlantic puffin (Fratercula arctica). Available at: https://jncc.gov.uk/our-work/atlantic-puffin-fratercula-arctica/. Accessed on: 30 August 2022.
JNCC. (2022a). Seabird Censuses. Available at: https://jncc.gov.uk/our-work/seabird-censuses/#previous-censuses-seabird-2000-1998-2002. Accessed on: 6 July 2022.
JNCC. (2022b). Seabirds Count – the fourth Breeding Seabird Census. Available at: https://jncc.gov.uk/our-work/seabirds-count/. Accessed on: 6 July 2022.
Johnston, A., Ausden, M., Dodd, A. M., Bradbury, R. B., Chamberlain, D. E., Jiguet, F., Thomas, C.D., Cook, A. S. C. P., Newson, S. E., Ockendon, N., Rehfisch, M. M., Roos, S., Thaxter, C. B., Brown, A., Crick, H. Q. P., Douse, A., McCall, R. A., Pontier, H., Stroud, D. A., Cadiou, B., Crowe, O., Deceuninck, B., Hornman, M. and Pearce-Higgins, J. W. (2013). Observed and predicted effects of climate change on species abundance in protected areas. Nature Climate Change, 93(3), pp.1055–1061.
Johnston, A., Cook, A.S., Wright, L.J., Humphreys, E.M. and Burton, N.H. (2014). Modelling flight heights of marine birds to more accurately assess collision risk with offshore wind turbines. Journal of Applied Ecology, 51(1), pp.31-41.
Johnston, D.T., Humphreys, E.M., Davies, J.G. and Pearce-Higgins, J.W. (2021). Review of climate change mechanisms affecting seabirds within the INTERREG VA area. Report to Agri-Food and Biosciences Institute and Marine Scotland Science as part of the Marine Protected Area Management and Monitoring (MarPAMM) project.
Jongbloed, R.H. (2016) Flight height of seabirds. A literature study. IMARES. Report C024/16.
Kadin, M., Österblom, H., Hentati-Sundberg, J. and Olsson, O. (2012). Contrasting effects of food quality and quantity on a marine top predator. Marine Ecology Progress Series, 444, pp.239–249.
Kaldellis, J.K. and Apostolou, D. (2017). Life cycle energy and carbon footprint of offshore wind energy. Comparison with onshore counterpart. Renewable Energy, 108, pp.72-84.
Kerckhof, F., Degraer, S., Norro, A. and Rumes. B. (2011). Offshore intertidal hard substrata: a new habitat promoting non-indigenous species in the Southern North Sea: an exploratory study. Offshore wind farms in the Belgian Part of the North Sea: Selected findings from the baseline and targeted monitoring, pp. 27-37.
Kober, K., Webb, A., Win, I., Lewis, M., O’Brien, S., Wilson, L.J., and Reid, J.B. (2010). JNCC Report No: 431, An analysis of the numbers and distribution of seabirds within the British Fishery Limit aimed at identifying areas that qualify as possible marine SPAs. Joint Nature Conservation Committee, ISSN 0963-8091.
Kober, K., Wilson, L.J., Black, J., O’Brien, S., Allen, S., Win, I., Bingham, C. and Reid, J.B. (2012). The identification of possible marine SPAs for seabirds in the UK: The application of Stage 1.1-1.4 of the SPA selection guidelines. Joint Nature Conservation Committee, ISSN 0963-8091.
Kogure, Y., Sato, K., Watanuki, Y., Wanless, S. and Daunt, F. (2016). European shags optimize their flight behavior according to wind conditions. Journal of Experimental Biology, 219, pp.311-318.
Krijgsveld, K.L. (2014). Avoidance behaviour of birds around offshore wind farms. Overview of knowledge including effects of configuration. Rapport Bureau Waardenburg, pp.13-268.
Krijgsveld, K.L., Fijn, R.C., Japink, M., van Horssen, P.W., Heunks, C., Collier, M.P., Poot, M.J.M., Beuker, D. and Dirksen, S. (2011). Effect Studies Offshore Wind Farm Egmond aan Zee. Final report on fluxes, flight altitudes and behaviour of flying birds. Bureau Waardenburg report 10-219, NZW-Report R231T1 flux and flight. Bureau Waardenburg, Culmeborg, Netherlands.
Kubetzki and Garthe. (2003). Distribution, diet and habitat selection by four sympatrically breeding gull species in the south-eastern North Sea. Marine Biology, 143(1), pp.199-207.
Lahoz-Monfort, J., Morgan, B. J. T., Harris, M. P., Wanless, S. and Freeman, S. N. (2011). A capture–recapture model for exploring multi-species synchrony in survival. Methods in Ecology and Evolution, 2(1), pp.116,124.
Lane, J.V., Jeavons, R., Deakin, Z., Sherley, R.B., Pollock, C.J., Wanless, R.J., and Hamer, K.C. (2020). Vulnerability of northern gannets to offshore windfarms; seasonal and sex-specific collision risk and demographic consequences. Marine Environmental Research, 162, 105196.
Lane, J. V., Spracklen, D. and Hamer, K.C. (2019). Effects of windscape on three-dimensional foraging behaviour in a wide-ranging marine predator, the northern gannet. Marine Ecology Progress Series, 628, pp.183–193
Lane, M.A., Walawender, M., Carter, J., Brownsword, E.A., Landay, T., Gillespie, T.R., Fairley, J.K., Philipsborn, R. and Kraft, C.S. (2022). Climate change and influenza: A scoping review. The Journal of Climate Change and Health, 5, 100084.
Larsen, F., Kindt-Larsen, L., Sørensen, T. K. and Glemarec, G. (2021). Bycatch of marine mammals and seabirds – occurrence and mitigation. DTU Aqua Report no 389-2021.
Lauria, V., Attrill, M.J., Brown, A., Edwards, M. and Votier, S.C. (2013). Regional variation in the impact of climate change: evidence that bottom-up regulation from plankton to seabirds is weak in parts of the Northeast Atlantic. Marine Ecology Progress Series, 488, pp.11-22.
Lauria, V., Attrill, M.J., Pinnegar, J.K., Brown, A., Edwards, M. and Votier, S.C. (2012). Influence of Climate Change and Trophic Coupling across Four Trophic Levels in the Celtic Sea. PLoS ONE, 7, e47408.
Le Bot, T., Lescroel, A., Fort, J., Péron, C., Gimenez, O., Provost, P., and Gremillet, D. (2019). Fishery discards do not compensate natural prey shortage in Northern gannets from the English Channel. Biological conservation, 236, pp.375-384.
Lean, F., Vitores, A. G., Reid, S. M., Banyard, A. C, Brown, H. I., Núñez, A. and Hansen, R. D. E. (2022). Gross pathology of high pathogenicity avian influenza virus H5N1 2021–2022 epizootic in naturally infected birds in the United Kingdom. One Health, 14 (2).
Letcher, R. J., Bustnes, J. O., Dietz, R., Jenssen, B. M., Jorgensen, E. H., Sonne, C., Verreault, J., Vijayan, M. M. and Gabrielsen, G. W. (2010) Exposure and effects assessment of persistent organohalogen contaminants in arctic 9 wildlife and fish. Science of The Total Environment, 408, pp.2995-3043.
Leopold, M., van Bemmelen, R. and Zuur, A. (2013). Responses of Local Birds to the Offshore Wind Farms PAWP and OWEZ off the Dutch mainland coast (Report No. C151/12). Report by IMARES - Wageningen UR.
Leopold, M.F., Dijkman, E.M., Teal, L. and OWEZ-Team. (2011). Local birds in and around offshore wind farm Egmond aan ZEE (OWEZ) (T-0 and T-1, 2002-2010). IMARES Wageningen UR.
Lewandowska, A., and Sommer, U. (2010). Climate change and the spring bloom: A mesocosm study on the influence of light and temperature on phytoplankton and mesozooplankton. Marine Ecology Progress Series, 405, pp.101-111.
Lewis, S., Sherratt, K. C., Hamer, M. P. and Wanless, S. (2003). Contrasting diet quality of northern gannets Morus bassanus at two colonies. Ardea, 91(2), pp.167-175.
Lewis, S., Wanless, S., Wright, P.J., Harris, M.P., Bull, J. and Elston, D.A. (2001). Diet and breeding performance of black-legged kittiwakes Rissa tridactyla at a North Sea colony. Marine Ecology Progress Series, 221, pp.277-284.
Lewison, R.L., Crowder, L.B., Wallace, B.P., Moore, J.E., Cox, T., Zydelis, R., McDonald, S., DiMatteo, A., Dunn, D.C., Kot, C.Y. and Bjorkland, R. (2014). Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proceedings of the National Academy of Sciences, 111(14), pp.5271-5276.
Linley, E.A.S., Wilding, T.A., Black, K., Hawkins, A.J.S. and Mangi S. (2007) Review of the Reef Effects of Offshore Wind Farm Structures and their Potential for Enhancement and Mitigation. Report from PML Applications Ltd and the Scottish Association for Marine Science to the Department for Business, Enterprise and Regulatory Reform (BERR), Contract No: RFCA/005/0029P.
Litzow, M. A., Piat, J. F., Prichard, A. K. and Roby, D. D. (2002) Response of pigeon guillemots to variable abundance of high-lipid and low-lipid prey. Oecologia, 132, pp.286–295.
Lindegren, M., Van Deurs, M., MacKenzie, B.R., Worsoe Clausen, L., Christensen, A. and Rindorf (2018). Productivity and recovery of forage fish under climate change and fishing: North Sea sandeel as a case study. Fisheries Oceanography, 27(3), pp.212-221.
Løkkeborg, S. (2011). Best practices to mitigate seabird bycatch in longline, trawl and gillnet fisheries—efficiency and practical applicability. Marine Ecology Progress Series, 435, pp.285-303.
Louzao, M., Gallagher, R., García-Barón, I., Chust, G., Intxausti, I., Albisu, J., Brereton, T. and Fontán, A. (2019). Threshold responses in bird mortality driven by extreme wind events. Ecological Indicators, 99, pp.183-192.
Lynam, C. P., Llope, M., Möllmann, C., Helaouët, P., Bayliss-Brown, G. A., and Stenseth, N. C. (2017). Interaction between top-down and bottom-up control in marine food webs. Proceedings of the National Academy of Sciences, 114(8), pp.1952-1957.
Maar, M., Bolding, K., Petersen, J. K., Hansen, J. L. and Timmermann, K. (2009). Local effects of blue mussels around turbine foundations in an ecosystem model of Nysted off-shore wind farm, Denmark. Journal of Sea Research, 62(2-3), pp.159-174.
MacDonald, A., Heath, M., Edwards, M., Furness, R., Pinnegar, J.K., Wanless, S., Speirs, D. and Greenstreet, S. (2015). Climate driven trophic cascades affecting seabirds around the British Isles. Oceanography and Marine Biology - An Annual Review, 53, pp.55-80.
MacDonald, A., Spiers, D.C., Greenstreet, S.P.R., Boulcott, P. and Heath, M.R. (2019). Trends in Sandeel Growth and Abundance off the East Coast of Scotland. Frontiers in Marine Science, 6, pp.201.
Mackas, D.L., and Beaugrand, G. (2010). Comparisons of zooplankton time series. Journal of Marine Systems, 79(3–4), pp.286-304.
Mackinson, S. and Daskalov, G. (2007). An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation. Science Services Technical Report no 142, Cefas Lowestoft, 142, pp.196
Mainwaring, M. C., Barber, I., Deeming, D. C., Pike, D. A., Roznik, E. A. and Hartley, I. R. (2016) Climate change and nesting behaviour in vertebrates: a review of the ecological threats and potential for adaptive responses. Biological Reviews, 92(4), pp.1991-2002.
Mallory, M.L., Gaston, A.J. and Gilchrist, H.G. (2009). Sources of breeding season mortality in Canadian Arctic seabirds. Arctic, pp.333-341.
Marine Management Organisation. (2019) Landing oblication general requirements. Available at: https://www.gov.uk/government/publications/landing-obligation-2019-rules-and-regulations/landing-obligation-general-requirements-2019--2v
Marine Scotland. (2020). Seabirds. Available at: https://marine.gov.scot/sma/assessment/seabirds-0. Accessed on: 20 August 2022.
Martin, G., Becker, D. J. and Plowright, R. K. (2018). Environmental Persistence of Influenza H5N1 Is Driven by Temperature and Salinity: Insights From a Bayesian Meta-Analysis. Frontiers in Ecology and Evolution, 6, pp.131.
Martin, M. (2022). RSPB Avian Influenza update. RSPB. Available at: https://community.rspb.org.uk/ourwork/b/scotland/posts/avian-influenza-update Accessed on: 19 July 2022.
Masden, E.A., Haydon, D.T. Fox, A.D. and Furness, R.W. (2010). Barriers to movement: modelling energetic costs of avoiding marine wind farms amongst breeding seabirds. Marine Pollution Bulletin, 60, pp.1085-1091.
Mavraki, N., Degraer, S. and Vanaverbeke, J. (2021) Offshore wind farms and the attraction–production hypothesis: insights from a combination of stomach content and stable isotope analyses. Hydrobiologia, 848, pp.1639-1657.
McCarthy, G.D., Jackson, L.C., Cunningham, S.A., Holliday. N.P., Smeed, D.A and Stevens, D.P. (2020). Effects of climate change on the Atlantic Heat Conveyor relevant to the UK. Marine Climate Change Impacts Partnership Science Review 2020, 190-207.
Marine Climate Change Impacts Partnership (MCCIP). (2018). Sandeels and their availability as seabird prey. MCCIP. pp.2-5.
McGinty, N., Barton, A.D., Record, N.R., Finkel, Z.V., Johns, D.G., Stock, C.A. and Irwin, A.J. (2021). Anthropogenic climate change impacts on copepod trait biogeography. Global Change Biology, 27(7), pp.1431-1442.
Mendiola, D., Alvarez, P., Cotano, U. and Martínezde Murguía, A. (2007) Early development and growth of the laboratory reared north-east Atlantic mackerel Scomber scombrus. Journal of Fish Biology, 70, pp.911–933.
Merkel, B. (2019). Migration in seabirds: seasonal structure in space and environment across species, populations and individuals. PhD Thesis. The Arctic University of Norway.
Merrick, R. L., Chumbley, M.K. and Byrd, G.V. (1997). Diet diversity of Steller sea lions (Eumetopias jubatus) and their population decline in Alaska: a potential relationship. Canadian Journal of Fisheries and Aquatic Sciences, 54, pp.1342–1348.
Mesquita, M.d.S., Erikstad, K.E., Sandvik, H., Barrett, R.T., Reiertsen, T.K., Anker-Nilssen, T., Hodges, K.I. and Bader, J. (2015). There is more to climate than the North Atlantic Oscillation: a new perspective from climate dynamics to explain the variability in population growth rates of a long-lived seabird. Frontiers in Ecology and Evolution, 3(43).
Miller, M.E., Hamann, M. and Kroon, F.J. (2020). Bioaccumulation and biomagnification of microplastics in marine organisms: a review and meta-analysis of current data. PLoS ONE, 15(10), e0240792.
Mitchell, I., Aonghais Cook, A., Douse, A., Foster, S., Kershaw, M., Neil McCulloch, N., Murphy, M. and Hawkridge, J. (2018). Marine Bird Breeding Success/failure. UK Marine Online Assessment Tool. Available at: https://moat.cefas.co.uk/biodiversity-food-webs-and-marine-protected-areas/birds/abundance/. Accessed on: 20 August 2022.
Mitchell, I., Daunt, F., Frederiksen, M. and Wade, K. (2020). Impacts of climate change on seabirds, relevant to the coastal and marine environment around the UK. MCCIP Science Review 2020, pp.382-399.
Mitchell, I., Daunt, F., Frederiksen, M. and Wade, K. (2020). Impacts of climate change on seabirds, relevant to the coastal and marine environment around the UK. Marine Climate Change Impacts Partnership Science Review, 382-399.
Mitchell, P.I., Newton, S.F., Ratcliffe, N. and Dunn, T.E. (2004). Seabird populations of Britain and Ireland. T. and AD Poyser, London.
Montevecchi, B., Chardine, J., Rail, J.-F., Garthe, S., Pelletier, D., Regular, P., Burke, C., Hedd, A., McFarlane Tranquilla, L., Bennett, S., Mooney, C., Power, K., Power, T., Hogan, H., Daoust, P.-Y., Lawson, J., Rogers, L., Wilhelm, S., Montevecchi M. and Lang, A. (2013). Extreme event in a changing ocean climate: Warm-water perturbation of 2012 influences breeding gannets and other marine animals in the northwest Atlantic and Gulf of St. Lawrence. The Osprey, 44, pp.14–19.
Morley, T.I., Fayet, A.L., Jessop, H., Veron, P., Veron, M., Clark, J. and Wood, M.J. (2016).The seabird wreck in the Bay of Biscay and the Southwest Approaches in 2014: a review of reported mortality. Seabird, 29, 22-38.
Murray, S., Harris, M. P., and Wanless, S. (2015). The status of Gannet in Scotland in 2013-2014. Scottish Birds, 35(1), pp.3-18.
NatureScot. (2018). Scottish Biodiversity Indicator – The Numbers and Breeding Success of Seabirds (1986 to 2019). Available at: www.nature.scot/doc/scottish-biodiversity-indicator-numbers-and-breeding-success-seabirds-1986-2019. Accessed on 15 July 2022.
NatureScot. (2020). Citation for Special Protection Area (SPA) Site Description: Outer Firth Of Forth and St Andrews Bay Complex (UK9020316). NatureScot.
NatureScot. (2022a). Island nature reserves close to protect seabirds. Available at: https://www.nature.scot/island-nature-reserves-close-protect-seabirds. Accessed on: 15 August 2022.
NatureScot. (2022b). Marine non-native species. Available at: https://www.nature.scot/professional-advice/land-and-sea-management/managing-coasts-and-seas/marine-non-native-species. Accessed on: 22 September 2022.
Newell, M., Harris, M. P., Daunt, F., Watts, E., Quinn, L. and Wanless, S. (2013) Isle of May seabird studies in 2007. JNCC Peterborough, JNCC Report No. 475c.
Newell, M., Wanless, S., Harris, M. and Daunt, F. (2015). Effects of an extreme weather event on seabird breeding success at a North Sea colony. Marine Ecology Progress Series, 532, pp.257–268
Non-native Species Secretariat (NNSS). (2022). The Non-native Species Information Portal (NNSIP). Available at: https://www.nonnativespecies.org/non-native-species/information-portal/. Accessed on: 22 September 2022.
Northridge, S., Kingston, A. and Coram., A. (2020). Preliminary estimates of seabird bycatch by UK vessels in UK and adjacent waters. Report prepared for the Department for Environment Food and Rural Affairs (Project Code ME6024).
Orben, R.A., Paredes, R., Roby, D.D., Irons, D.B. and Shaffer, S.A. (2015) Wintering North Pacific black-legged kittiwakes balance spatial flexibility and consistency. Movement Ecology. 21(3), pp.36.
ORE Catapult. 2021. Carbon footprint of offshore wind farm components. Available at: https://ore.catapult.org.uk/wp-content/uploads/2021/04/Carbon-footprint-of-offshore-wind-farm-components_FINAL_AS-3.pdf. Accessed on: 13 August 2022.
OSPAR. (2017). Intermediate Assessment, 2017: Marine Bird Breeding Success/Failure. Available at: https://oap.ospar.org/en/ospar-assessments/intermediate-assessment-2017/biodiversity-status/marine-birds/marine-bird-breeding-success-failure/. Accessed on: 14 August 2022.
Oswald, S.A. and Arnold, J.M. (2012). Direct impacts of climatic warming on heat stress in endothermic species: seabirds as bioindicators of changing thermoregulatory constraints. Integrative Zoology, 7(2), pp.121-136.
Oswald, S.A., Bearhop, S., Furness, R.W., Huntley, B. and Hmer, K.C. (2008). Heat stress in a high-latitute seabird: effects of temperature and food supply on bathing and nest attendance of great skuas Catharacta skua. Journal of Avian Biology, 39(2), pp.163-169.
Paleczny, M., Hammill, E., Karpouzi, V. and Pauly, D. (2015). Population trend of the world’s monitored seabirds, 1950-2010. PLoS ONE, 10, e0129342.
Pashby, B.S. and Cudworth, J. (1969). The fulmar ‘wreck’ of 1962. Available at: https://britishbirds.co.uk/wp-content/uploads/article_files/V62/V62_N03/V62_N03_P097_109_A022.pdf#:~:text=An%20appeal%20for%20information%20regarding%20dead%20Fulmars%20in,north-west%20Europe%2C%20notably%20in%20the%20Netherlands%20and%20Sweden. Accessed on: 26 August 2022.
Peschko, V., Mendel, B., Mercker, M., Dierschke, J. and Garthe, S. (2021). Northern gannets (Morus bassanus) are strongly affected by operating offshore wind farms during the breeding season. Journal of Environmental Management, 279, 111509.
Peschko, V., Mercker, M., and Garthe, S. (2020). Telemetry reveals strong effects of ofshore wind farms on behaviour and habitat use of common guillemots (Uria aalge) during the breeding season. Marine Biology, 167, 118.
Petersen, J.K. and Malm, T. (2006). Offshore windmill farms: threats to or possibilities for the marine environment. AMBIO: A Journal of the Human Environment, 35, pp.75-80.
Philip, E. and Tyler, G. (2022) Weathering the storm: A policy Update. The 15th International Seabird Group Conference, Cork Ireland, seabird conference cork, 22nd to 25th August 2022.
Piatt, J.F. and Anderson, P. (1996). Response of common murres to the Exxon Valdez oil spill and long-term changes in the Gulf of Alaska marine ecosystem. American Fisheries Society Symposium. 18, pp720–737
Piatt, J. F and Nettleship, D. N. (1985). Diving depths of four alcids. The Auk. 102, pp.293-297.
Pierotti, R. J. and Good, T. P. (1994) Herring Gull (Larus argentatus) in The Birds of North America, No. 124. Philadelphia: The Academy of Natural Sciences; Washington, D.C.
Plastics Europe. (2021). Plastics–the facts 2021. Plastics Europe, pp.1-64.
Provencher, J.F., Gaston, A.J., Mallory, M.L., O’hara, P.D. and Gilchrist, H.G. (2010). Ingested plastic in a diving seabird, the thick-billed murre (Uria lomvia), in the eastern Canadian Arctic. Marine Pollution Bulletin, 60(9), pp.1406-1411.
Purcell, J. E, Uye, S. and Lo, W. (2007) Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Available at: https://www.researchgate.net/publication/234155125_Anthropogenic_cause_of_jellyfish_blooms_and_their_direct_consequences_for_humans_A_review#:~:text=Jellyfish%20are%20infamous%20for%20their,clogging%20cooling%2Dwater%20intake%20screens. Accessed on: 20 August 2022.
Rahmstorf, S. and Coumou, D. (2011). Increase of extreme events in a warming world. Proceedings of the National Academy of Science, 108, 17905–17909.
Ramos, J. A. and Furness, R. W. (2022). Seabirds as Indicators of Forage Fish Stocks. CRC Press. ISBN 9781003047520.
Ratcliffe, N., Schmitt, S., Mayo, A., Tratalos, J. and Drewitt, A. (2008). Colony habitat selection by little terms Sternula albifrons in East Anglia: implications for coastal management. Seabird, 21, pp.55-63.
Réginer, T., Gibb, F T., and Wright, P. J. (2017). Importance of trophic mismatch in a winter- hatching species: evidence from lesser sandeel. https://www.int-res.com/abstracts/meps/v567/p185-197/
Reubens, J.T., Degraer, S. and Vincx, M. (2014). The ecology of benthopelagic fishes at offshore wind farms: a synthesis of 4 years of research. Hydrobiologia, 727, pp.121–136.
Rice, J. (1995). Food web theory, marine food webs, and what climate change may do to northern marine fish populations. Pages 561–568 in R. J. Beamish, ed. Climate change and northern fish populations. Can. Spec. Publ. Fish. Aquat. Sci., 121.
Richier, S., Achterberg, E.P., Dumousseaud, C., Poulton, A.J., Suggett, D.J., Tyrrell, T., Zubkov, and Moore, C. M. (2014). Phytoplankton responses and associated carbon cycling during shipboard carbonate chemistry manipulation experiments conducted around Northwest European shelf seas. Biogeosciences, 11, pp.4733-4752.
Riebesall, U., Gattuso, J., Thinsgstad, T.F. and Middelburg, J.J. (2013). Preface arctic ocean acidification: Pelagic ecosystem and biogeochemical responses during a mesocosm study. Biogeosciences, 10(8), pp.5619-5626.
Rindorf, A., Wanless, S. and Harris, M.P. (2000). Effects of changes in sandeel availability on the reproductive output of seabirds. Marine Ecology Progress Series, 202, pp.241–252.
Roberts, C.M., O’Leary, B.C., McCauley, D.J., Cury, P.M., Duarte, C.M., Lubchenco, J., Pauly, D., Sáenz-Arroyo, A., Sumaila, U.R., Wilson, R.W. and Worm, B. (2017). Marine reserves can mitigate and promote adaptation to climate change. Proceedings of the National Academy of Sciences, 114(24), pp.6167-6175.
Rock, P. and Vaughan, I.P. (2013). Long-term estimates of adult survival rates of urban Herring Gulls Larus argentatus and Lesser Black-backed Gulls Larus fuscus. Ringing and Migration, 28, pp.21–29.
Roman, L., Bryan, S., Bool, N., Gustafson, L. and Townsend, K. (2021). Desperate times call for desperate measures: non-food ingestion by starving seabirds. Marine Ecology Progress Series, 662, 157-168.
Roman, L., Hardesty, B.D., Hindell, M.A. and Wilcox, C. (2019). A quantitative analysis linking seabird mortality and marine debris ingestion. Scientific Reports, 9(1), pp.1-7.
Ropert-Coudert, Y., Daunt, F., Kato, A., Ryan, P.G., Lewis, S., Kobayashi, K., Grémillet, D. and Wanless, S. (2009). Underwater Wingbeats Extend Depth and Duration of Plunge Dives in Northern Gannets Morus Bassanus. Journal of Avian Biology, 40(4), pp.380.
Rosen, D. A. S. and Trites, A. W. (2000) Pollock and the decline of Steller sea lions: testing the junk-food hypothesis. Canadian Journal of Zoology, 78, pp.1243–1250.
Royal Society of the Protection of Birds (RSPB). (2022). Powering Healthy Seas: Accelerating Nature Positive Offshore Wind. A RSPB commissioned report. Available at: https://www.rspb.org.uk/globalassets/downloads/pa-documents/powering-healthy-seas-report_rspb_august-2022.pdf. Accessed on: 22 September 2022.
Russell, D.J.F., Wanless, S., Collingham, Y.C. and Hamer, K.C. (2015) Predicting future European breeding distributions of British seabird species under climate change and unlimited/no dispersal scenarios. Diversity, 7, pp.342-359.
Sadykova, D., Scott, B.E., De Dominicis, M., Wakelin, S.L., Wolf, J. and Sadykov, A. (2018). Ecological costs of climate change on marine predator-prey population distributions by 2050. Ecology and Evolution, 10, pp.1069-1086.
Sadykova, D., Scott, B.E., De Dominicis, M., Wakelin, S.L., Wolf, J. and Sadykov, A. (2020). Ecological costs of climate change on marine predator–prey population distributions by 2050. Ecology and evolution, 10(2), pp.1069-1086.
Schraft, H.A., Whelan, S. and Elliott, K.H. (2019). Huffin and puffin: seabirds use large bills to dissipate heat from energetically demanding flight. Journal of Experimental Biology, 222(21), jeb212563.
Schrum, C., Lowe, J., Markus Meier, H.E., Grabemann, I., Holt, J., Mathis, M., Pohlmann, T., Skogen, M.D., Sterl, A. and Wakelin, S. (2016). Projected Change—North Sea. In: Quante, M., Colijn, F. (eds) North Sea Region Climate Change Assessment. Regional Climate Studies. Springer, Cham.
Schutter, M., Dorenbosch, M., Driessen, F.M., Lengkeek, W., Bos, O.G. and Coolen, J.W. (2019). Oil and gas platforms as artificial substrates for epibenthic North Sea fauna: Effects of location and depth. Journal of Sea Research, 153, 101782.
Schwemmer, H., Schwemmer, P., Ehrich, S. and Garthe, S. (2013). Lesser black-backed gulls (Larus fuscus) consuming swimming crabs: An important link in the food web of the southern North Sea. Estuarine, Coastal and Shelf Science, 119, pp.71–78.
Scott, B. (2022). Ecologically Sustainable Futures for Large-scale Renewables and How to Get There. International Marine Energy Journal, 5(1), pp.37-43.
Scottish Government (2021) Energy strategy: position statement. Available at: https://www.gov.scot/publications/scotlands-energy-strategy-position-statement/pages/2/. Accessed on: 10 August 2022.
Scottish Government. (2015). National Marine Plan. Available at: https://www.gov.scot/publications/scotlands-national-marine-plan/pages/12/. Accessed on: 10 August 2022.
Scottish Government. (2017). The future of energy in Scotland: Scottish energy strategy. Available at: https://www.gov.scot/publications/scottish-energy-strategy-future-energy-scotland-9781788515276/. Accessed on: 10 August 2022.
Scottish Government. (2020a). Securing a green recovery on a path to net zero: climate change plan 2018–2032 – update. Available at: https://www.gov.scot/publications/securing-green-recovery-path-net-zero-update-climate-change-plan-20182032/pages/3/. Accessed on: 10 August 2022.
Scottish Government. (2020b). Sectoral marine plan for offshore wind energy. Available at: https://www.gov.scot/publications/sectoral-marine-plan-offshore-wind-energy/pages/2/. Accessed on: 10 August 2022.
SEAPOP. (2022). Auk wreck last autumn struck mainly young common guillemots. Available at: https://seapop.no/en/2022/02/unge-lomvier-rammet-av-omfattende-massedod-hosten-2021/#:~:text=In%20the%20autumn%20of%202021%2C%20a%20large%20number,building%2C%20extra%20attention%20is%20drawn%20and%20concern%20raised. Accessed on: 23 August 2022.
Searle, K.R., Waggitt, J., Evans, P., Bogdanova, M., Daunt, F. and Butler, A. (2022). Study to examine the impact of climate change on seabird species off the east coast of Scotland and potential implications for environmental assessments. Marine Scotland Science Report.
Shepard, E. (2021). Seabirds: When storm riders get wrecked. Current Biology, 31, R1035-R1063.
Sherley, R.B., Barham, B. J, Barham, P. J., Campbell, K. J., Crawford, R. J. M., Grigg, J., Howswill, C., McInnes, A., Morris, T. L., Pichegru, L. and Steinfurth, A. (2018). Bayesian inference reveals positive but subtle effects of experimental fishery closures on marine predator demographics. Proceedings of the Royal Society B: Biological Sciences, 285, 20172443.
Skov, H., Heinänen, S., Norman, T., Ward, R.M., Méndez-Roldán, S. and Ellis, I. (2018). ORJIP Bird Collision and Avoidance Study. Final report – April 2018. The Carbon Trust. United Kingdom, 247Skov H., Leonhard, S.B., Heinänen, S., Zydelis, R., Jensen, N.E., Durinck, J., Johansen, T.W., Jensen, B.P., Hansen, B.L., Piper, W. and Grøn, P.N. (2012). Horns Rev 2 Monitoring 2010-2012. Migrating Birds. Orbicon, DHI, Marine Observers and Biola. Report commissioned by DONG Energy.
Slavik, K., Lemmen, C., Zhang, W., Kerimoglu, O., Klingbeil, K. and Wirtz, K.W. (2019). The large-scale impact of offshore wind farm structures on pelagic primary productivity in the southern North Sea. Hydrobiologia, 845, pp.35-53
Stienen, E.W.M., Courtens, W., Van de walle, M., Vanermen, N. and Verstraete, H. (2017). Long-term monitoring study of beached seabirds shows that chronic oil pollution in the southern North Sea has almost halted. Marine Pollution Bulletin, 115, 194-200.
Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M.A. and Watanuki, Y. (2013). Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Marine pollution bulletin, 69(1-2), pp.219-222.
Tasker, M.L., Camphuysen, C.J., Cooper, J., Garthe, S., Montevecchi, W.A. and Blaber, S.J. (2000). The impacts of fishing on marine birds. ICES journal of Marine Science, 57(3), pp.531-547.
Tian, H., Dong, L., Boeckel, T. V and Pei, Y. (2014). Climate change suggests a shift of H5N1 risk in migratory birds. Ecological Modelling.
Troisi, G., Barton, S. and Bexton, S. (2016). Impacts of oil spills on seabirds: Unsustainable impacts of non-renewable energy. International Journal of Hydrogen Energy, 41(37), pp.1-7.
Tyson, C., Shamoun-Baranes, J., Van Loon, E. E., Camphuysen, K. C. J. and Hintzen, N. T. (2015). Individual specialization on fishery discards by lesser black-backed gulls (Larus fuscus). ICES Journal of Marine Science, 72, pp.1882–1891.
Uhlmann, S.S., Ulrich, C. and Kennelly, S.J. (2019). The European Landing Obligation: Reducing Discards in Complex, Multi-Species and Multi-Jurisdictional Fisheries. Springer Nature, 431.
UNEP. (2021). From Pollution to Solution: a global assessment of marine litter and plastic pollution. United Nations Environment Programme, Nairobi. Available at: https://www.unep.org/ resources/pollution-solution-global-assessment-marine-litter-and-plastic-pollution. Accessed on: 24 August 2022.
Van der Molen, J., Smith, H.C., Lepper, P., Limpenny, S. and Rees, J. (2014). Predicting the large-scale consequences of offshore wind turbine array development on a North Sea ecosystem. Continental shelf research, 85, pp.60-72.
Vanermen, N., Stienen, E.W.M., Courtens, W., Onkelinx, T., Van de walle, M. and Verstraete, H. (2013). Bird monitoring at offshore wind farms in the Belgian part of the North Sea - Assessing seabird displacement effects. Rapporten van het Instituut voor Natuur- en Bosonderzoek 2013 (INBO.R.2013.755887). Instituut voor Natuur- en Bosonderzoek, Brussel.
Verbeek, N. A. M. (1977). Comparative feeding behaviour of immature and adult herring gulls. The Wilson Bulletin, 89(3), pp.415-421.
Visser, M.E. and Gienapp, P. (2019). Evolutionary and demographic consequences of phenological mismatches. Nature Ecology and Evolution, 3, pp.879-885
Votier, S. C., Archibald, K., Morgan, G. and Morgan, L. (2011) The use of plastic debris as nesting material by a 22 colonial seabird and associated entanglement mortality. Marine Pollution Bulletin, 62, pp.168-172
Votier, S. C., Bearhop, S., Witt, M. J., Inger, R., Thompson, D. and Newton, J. (2010). Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. Journal of Applied Ecology, 47(2), pp.487-497.
Votier, S. C., Hatchwell, B. J., Beckerman, A., McCleery, R. H., Hunter, F. M., Pellatt, J., Trinder, M. and Birkhead, T. R. (2005). Oil pollution and climate have wide-scale impacts on seabird demographics. Ecology Letters, 8(11), pp.1157-1164.
Walther, G-R. (2010). Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society Biology, 365(1549), pp.2019-2024.
Wanless, S., Harris, M. P., Newell, M. A., Speakman, J. R. and Daunt, F. (2018). Community-wide decline in the occurrence of lesser sandeels Ammodytes marinus in seabird chick diets at a North Sea colony. Marine Ecology Progress Series, 600. 193-206.
Wanless, S., Harris, M. P., Redman, P. and Speakman, J. R. (2005). Low energy values of fish as a probable cause of major breading seabird failure in the North Sea. Marine Ecology Progress Series, 294, pp.1-8.
Wanless, S., Wright. P.J., Harris, M.P. and Elston, D.A. (2004). Evidence for decrease in size of lesser sandeels Ammodytes marinus in a North Sea aggregation over a 30-yr period. Mar Ecol Prog Ser, 279, pp.237-246.
Wilcox, C., Van Sebille, E. and Hardesty, B.D. (2015). Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proceedings of the national academy of sciences, 112(38), pp.11899-11904.
WindEurope. (2021). Offshore Wind in Europe: Key trends and statistics 2020. WindEurope. Available at: https://windeurope.org/intelligence-platform/product/offshore-wind-in-europe-key-trends-and-statistics-2020/#:~:text=Europe%20added%202.9%20GW%20of,wind%20turbines%20across%2012%20countries. Accessed on: 05 August 2022.
Wolf, J., Woolf, D. and Bricheno, L. (2020). Impacts of climate change on storms and waves relevant to the coastal and marine environment around the UK. MCCIP Science Review, 2020, pp.132-157.
Wright, P.J. and Bailey, M.C. (1996). Timing of hatching in Ammodytes marinus from Shetland waters and its significance to early growth and survivorship. Marine Biology, 126, 143-152.
Wright, P.J., Orpwood, J.E. and Scott, B.E. (2017). Impact of rising temperature on reproductive investment in a capital breeder: the lesser sandeel. Journal of Experimental Marine Biology and Ecology, 486, pp.52-58.
Ybema, M.S., Gloe, D. and Hille Ris Lambers, R. (2009). OWEZ-pelagic fish: progression after T1. IMARES Wageningen UR Report.
Zijl, F., Laan, S.C., Emmanouil, A., van Kessel, T., van Zelst, V.T.M., Vilmin, L.M. and van Duren, L.A. (2021). Potential ecosystem effects of large upscaling of offshore wind in the North Sea – Bottom-up approach. Deltares Report.
Zintzen, V. (2007). Biodiversity of shipwrecks from the Southern Bight of the North Sea. PhD thesis, Universite Catholique de Louvain, Leuven: 337.
Žydelis, R., Lewison, R.L., Shaffer, S.A., Moore, J.E., Boustany, A.M., Roberts, J.J., Sims, M., Dunn, D.C., Best, B.D., Tremblay, Y., and Kappes, M.A. (2011). Dynamic habitat models: using telemetry data to project fisheries bycatch. Proceedings of the Royal Society of London B: Biological Sciences, 278(1722), pp.3191-3200.
Žydelis, R., Small, C. and French, G. (2013). The incidental catch of seabirds in gillnet fisheries: A global review. Biological Conservation, 162, pp.76-88.