Razorbill
- Razorbill numbers in Scotland increased from 1969/70 to 2001 but declined from 2001 to 2009, remaining low between 2009-2013. They then increased up until 2017 and have sharply declined since (JNCC 2021). However, uncertainty in some of the abundance estimates means that patterns should be treated with caution. However, the abundance of Razorbills at Handa appears broadly to follow this pattern (Figure 2.7 and Figure 2.8). The last full colony count from 2019 numbered 8,207 individuals and represents a decline of 52% from 2001 (JNCC 2021). However, there is some evidence of recovery, as the previous 2014 count of 5,047 individuals represented the lowest count since records began (Figure 3.6). The Razorbill population on Handa has declined significantly in comparison with other sites in The Minch, such as Mingulay and Berneray, where the population dropped by 24% and The Shiants, where the population remained stable. The losses experienced at Handa are more in line with those from Sheltland and Orkney, that is a decline in 46% from Fair Isle and in 59% from West Westray (JNCC 2021).
- Productivity data for Razorbill has not been gathered at Handa. However, for Scotland as a whole the situation mirrors that described for Guillemot with some colonies experiencing continuing low levels of productivity (e.g. Papa Westray, and Fair Isle) whilst others have been relatively successful (e.g. North Sutor, Isle of May).
Puffin
- Estimates from the JNCC Seabird Censuses suggest that there was an increase in the UK Puffin population of 19% between Operation Seafarer (1969-70) and Seabird 2000 with an increase of 13% estimated for Scotland (JNCC 2021). The logistical issues with gathering data on Puffins mean that few colonies conduct population counts annually. However, counts at Handa have been conducted routinely, meaning that they can be compared with data from other regularly counted locations (Figure 2.9 and Figure 2.10).
- All colonies appear to have increased up until 2001 or 2002, after which numbers then declined. Monitoring on the Isle of May showed that return rates in 2007 and 2008 were particularly low. Some sites such as Sule Skerry show a recent increase, although others such as the Isle of May and Fair Isle do not. It is of note that the Handa data appears to show a significant increase in Puffin numbers following rat eradication in 1997 (Figure 2.10). Figure 2.10 also shows some recovery post 2010, followed by a decline after 2016, which is coincident with significant increases in rats (see discussion above).
- Although productivity data for Handa has not been gathered for Puffin, it has been generally low in Scotland with particularly poor years in 2007 due to food shortage and in 1998 and 2004 due to unprecedented rainfall flooding burrows (JNCC 2021). However, over the last decade it has increased, and in 2018 and 2019 reached 0.74 and 0.69 chick per pair respectively, which is similar to values from the first decade of the monitoring period (JNCC 2021).
Source: Atlantic puffin (Fratercula arctica) | JNCC - Adviser to Government on Nature Conservation https://jncc.gov.uk/our-work/atlantic-puffin-fratercula-arctica/
Figure 2.9: Number of Atlantic Puffin AOB at three colonies in Scotland, 1986-2019.
Source: Database provided by SWT
Figure 2.10: All island counts of Atlantic Puffin (individuals) at Handa Island from 1995-2020.
Population trends of other seabird species
- Other species listed as features of Handa SPA include Northern Fulmar Fulmarus glacialis, Great Skua Stercorarius skua and Arctic Skua Stercorarius parasiticus. Productivity data is collected for these species. All island counts are conducted every 1-2 years for the skuas and every 5 years for Fulmar. The skua species have been studied and ringed under the Handa Island Skua Project. Other species that are routinely counted include European Shag Phalacrocorax aristotelis, Great Black-backed Gull Larus marinus, Herring Gull Larus argentatus. All island counts for Shag are conducted every few years, but for Herring Gull and Great Black-backed Gull are conducted annually. Common Tern Sterna hirundo and Arctic Tern Sterna paradisaea are also counted annually. Common Gull has been counted on a more casual basis, since it is not listed on either the SSSI or SPA designation. Other breeding species routinely monitored include Red Throated Diver Gavia stellata.
- Other breeding species are recorded on a more casual basis. Other species that routinely breed on Handa include (but are not limited to) Common Eider Somateria mollissima, Common Shelduck Tadorna tadorna, Eurasian Oystercatcher Haemotopus ostralegus, Ringed Plover Charadrius hiaticula, Common Snipe Gallinago gallinago and Wheatear Oenanthe oenanthe.
- Other species of interest that occur on Handa, but which have not been confirmed as breeding include Black Guillemot Cepphys grylle, which is still regularly sighted on Handa. Black Guillemot is known to have bred on Handa >100 years ago, and the introduction of Brown Rat, which is first mentioned by Harvey-Brown and Buckley in 1887, is blamed for their decline (Harvey-Brown & Buckley 1887). It was suspected that European Storm Petrel Hydrobates pelagicus may breed on Handa, and mist netting was carried out on several occasions throughout the early noughties resulting in the capture of several birds, some of which had brood patches (SWT 2005). However, it could not be confirmed whether these birds were breeding on Handa as Storm Petrel is a pelagic species which may occur almost anywhere. Storm Petrel has not been sighted in recent years; however, it is a cryptic species and without specific searches for them (i.e. mist netting and or/playing calls) it is not anticipated that they would necessarily be detected.
Fulmar
- Seabird census data shows that Fulmar increased between 1969/70 and 1985-88 but has since suffered a protracted decline (JNCC 2021). At Handa Fulmars reached a peak of 3,600 AOS in 1977, levelling off in 1979 at 3,000 AOS. The recent JNCC survey in 2017 shows that following a period of stability Fulmars are now in decline, with a loss of 60% between 2000 and 2017, that is a decline from 3,550 to 1,423 AOS (Figure 2.5 above, JNCC 2021). North Rona also recorded a similar decline of 59%, although other sites in The Minch such as Rathlin, and Mingulay and Berneray did not suffer such severe declines, with numbers at these sites declining by 25% and 1.4% respectively. Productivity for Handa in 2016 and 2019 was 0.56 and 0.42, higher than the Scottish average of 0.47 and 0.39 for these years.
Skuas
- Handa supports one of the largest colonies of Great Skua in the UK and is one of the few sites where numbers had been increasing (Figure 3.9). Both skua species started nesting on Handa in the 1960s. Since 2000 Arctic Skua has been undergoing a protracted decline and have decreased from 42 AOT in 2001 to only 20 AOT in 2019, with the primary cause believed to be predation pressure from Great Skua (Jones et al. 2008). This is characterised by relatively high productivity coupled with declining numbers (Figure 2.11 and Figure 2.12). Unlike Arctic Skua, Great Skua colonies rarely experience years of breeding failure, and it is thought that this is due to their ability to switch prey, often becoming cannibalistic and also preying on the chicks and adults of other seabird species. By contrast Arctic Skua relies on stealing fish from other species (kleptoparasitism), and therefore productivity was particularly poor from 2006-2008 due to lack of prey being delivered by their host species, namely auks and Kittiwake (Perkins et al. 2018). It is of interest that Handa also supports a ‘club’ of non-breeding Great Skua at Hill Loch.
Gulls
- Small numbers of Great Black-backed Gull, Herring Gull and Common Gull breed on Handa. Great Black-backed Gull is in decline, whereas Herring Gull numbers are more stable at ~10-15 AOT. Previously Handa has supported up to 418 pairs of Herring Gull and 62 pairs of Great Black-backed Gull (in 1977 and 1970 respectively) (Figure 2.13 and Figure 2.14 below, Stoneman & Willcox 1995). The Common Gull colony numbered 31-32 pairs in the early noughties (SWT 2003, SWT 2005), having roughly doubled after rat eradication in 1997. Numbers are now very low with the latest count numbering just 9 pairs (SWT 2020). Handa previously supported very low numbers of Lesser Black-backed Gull, though breeding has not been recorded since 1989 (Stoneman & Willcox 1995).
Source: Database provided by SWT
Figure 2.11: Great and Arctic Skua AOTs at Handa Island from 1995-2019.
Source: Database provided by SWT
Figure 2.12: Great and Arctic Skua productivity at Handa Island from 2003-2017.
Source: Database provided by SWT
Figure 2.13: Great Black-backed Gull AONs on Handa Island from 1998 to 2020.
Source: Database provided by SWT
Figure 2.14: Herring Gull AONs on Handa Island from 1998 to 2020.
Terns
- Arctic Terns were last confirmed as breeding on Handa in 2015, although in 2021 Arctic Terns were observed around Port an Eilein for the majority of the season (SWT 2021). In 2022 tern chicks and fledglings (most likely Arctic) were seen for the first time in 7 years. In the early 1990s numbers of terns on Handa were small with only 1-5 pairs of Arctic Terns and fewer than 8 pairs of Common Terns (Stoneman & Willcox 1995). Numbers increased significantly following rat eradication in 1997 reaching 22 Common Tern pairs and 33 Arctic Tern pairs in 2001.
- In the same year there was also a large post breeding aggregation of 250 post-breeding Arctic Terns which arrived in mid-July and stayed for several weeks (SWT 2001). However, a series of poor years followed with various issues affecting breeding performance from sheep disturbance, unseasonal storms and high winds and seemingly unexplained nest abandonment[16].
European Shag
- In the 1970s there were reported to be as many as 400 pairs of European Shag breeding on Handa (Stoneman & Willcox 1995). In 1998 there were ~120 AON, but numbers have since fallen significantly to just 16 AON in 2020 (Figure 2.15, SWT 1998, SWT 2020). A study showed that breeding numbers in Shetland declined in a similar manner by about 87% since Seabird 2000 and it has been suggested that the majority of decline could be attributed to prolonged strong winds making foraging difficult, resulting in starvation (Wanless et al. 2018).
Source: Database provided by SWT
Figure 2.15: European Shag AONs on Handa Island from 1998 to 2020.
2.4.2. Benefits of the 1997 rat eradiaction
- Following the eradication of rats from Handa in 1997 several positive changes were recorded, the most notable being the increase in Puffin numbers. This rise is apparent in Figure 2.10 Prior to rat eradication breeding Puffins had been restricted to nesting on Great Stack. In 1999, two years after rat eradication, 20-50 AOB were counted on the main island adjacent to Great Stack. Following rat eradication in 1997 Puffin numbers on Handa increased from ~500 individuals to a maximum of 735 individuals in 2001, a 47% increase in just 5 years (Figure 2.10). It is of note that although Puffins were increasing nationally during the period between 1969/70 and 2000, on Handa they had been declining. Therefore, rat eradication had the effect of not only halting the decline but also in bringing about a very substantial population increase during this 5-year period.
- In total, seven new Puffin nesting areas were identified between 1999-2003 (2.16 below, Stoneman & Zonfrillo 2005). One of the new nesting areas was located to the south-east of Great Stack above the cliff edge and below the tourist view-point. Control of visitors was recommended by SWT wardens in 2001 and implemented successfully in 2002 but the Puffins did not return to this area again. However, this, and several other areas are still fenced off so that Puffins can potentially recolonise. Use of the additional nesting areas is noted in 2003 but these areas are not referred to again in wardens’ reports. Puffin numbers in 2004 and 2005 were notably poor (Figure 2.10). A combination prey failures, hedgehogs and rat invasion in 2005 will have contributed to current trends of decline. In some locations there is evidence of some amelioration of declines in recent years, however, this does not appear to be the case with Handa (Figure 2.10).
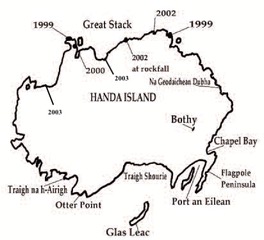
Source: Stoneman & Zonfrillo 2005 with additional nesting areas added from SWT 2003.
Figure 2.16: Map of Handa showing dates and locations of new Puffin colonies on the main island following the eradication of rats in 1997.
- Following rat eradication in 1997 Common and Arctic Terns increased, leading to some of the most successful breeding seasons ever recorded on Handa (Figure 2.17). Arctic Terns originally bred on the skerries at Port an Eilein, an islet that is cut off from the mainland at high tide. A small number were also reported to be breeding at Glas Leac, an adjacent small islet further offshore. In 1999 a new colony was established on the main island at Traigh Shourie.
- Common Gull also increased in the years following rat eradication. Initially there was a small colony of ~20 pairs at Otter Point. A new colony at Port an Eilein was observed in 1998, increasing the number of breeding pairs to ~31-32. The colony at Port an Eilein is now gone with just a very small number of pairs left at Otter Point
- A monitoring plot was established at the cliffs to investigate whether Fulmar chick survival would improve following rat eradication. Chicks were counted annually at the end of August (Figure 2.18). There was a significant rise in chicks fledged between 1999 and 2000, which could be associated with improved fledging success in the absence of rats, although this is difficult to establish due to the declines that followed.
Source: Stoneman & Zonfrillo 2005
Figure 2.17: Number of nesting Common and Arctic Terns on Handa between 1974 and 2004.
Source: SWT 2005.
- However, it was noted that following eradication there was a change in the distribution of Fulmar nests, with more nests before rat eradication on the north-west of the island, whilst after rat eradication there were more nests in the north and north-east sites. The number of nests increased in Fulmar Bay (SWT 1998), which is still in use as a nest site, although productivity from this breeding location is now notably poor (see Section 2.4.6).
- Other species were also recorded as breeding either for the first time or with increased success. These included Oystercatchers and Ringed Plovers, which were recorded fledging chicks for the first time (SWT 1998). New breeding birds for Handa included Shelduck, Redshank and Rock Pigeon, although numbers of all these were low. Sightings of Pygmy Shrew increased (SWT 1998, 1999, 2001). In 1998 extensive growth of sea rocket (Cakile maritima) and Orache (Atriplex spp.) were observed on all the islands beaches. It was thought that previously the rats consumed any sea rockets seeds. Annual growth of these species persisted until 2001, although the quantity of vegetation decreased due to grazing by sheep and the recovering rabbit population (with the latter perhaps also being a response to rat eradication).
- It was hoped that rat eradication would assist the return of Black Guillemot. Although prospecting birds were recorded in 1999-2001, this behaviour was not observed again.
- It was also hoped that eradication of rats would enable colonisation by European Storm Petrel. Although there are no records of this species breeding on Handa, it was thought that the habitat was suitable. Several searches were carried out for breeding Storm Petrels, including mist netting in 2003 and 2004. In 2004, 20% of the 96 birds netted had brood patches – one birds was a recapture from 2003. On this basis it was suggested that some birds may be breeding locally, although this was never confirmed (Stoneman & Zonfrillo 2005).
- In summary, some positive changes in seabird abundance and distribution were recorded between 1997-2003. However, the majority of species declined during the period between 2004-2008 due to large scale sandeel failures (MacDonald et al. 2019, Mitchell et al. 2020). Although rat eradiation may have alleviated the severity of the decline, the overall trend of decline was so severe that any positive effects of rat eradication are difficult to detect for many species. A similar phenomenon has occurred at Canna whereby rat eradication has had the effect of slowing the rate of decline for some species such as Guillemot and Kittiwake (Luxmoore et al. 2019). However, in recent years as prey availability seems to have ameliorated somewhat, then significant increases of both Kittiwake and Guillemot have been observed (The Seabird Group 2019).
2.4.3. Project description
- The rat eradication project at Handa is being developed in accordance with the UK Rodent Eradication Best Practice Toolkit (Thomas et al. 2017a). A brief outline of the work is included here, whilst further detail is located within the Implementation and Monitoring Plan. This includes the approach to developing a Biosecurity Plan, incorporating information on how incursion of invasive mammalian predators will be prevented, surveillance to monitor for incursion, and incursion response plans should an incursion occur.
- The difficulties in removing rats from Handa in 1997 and maintaining Handa as free from rats are significant. The number of incursions and invasions since initial rat eradication in 1997 show that maintaining Handa rat-free, or as near rat free as possible will be challenging.
- However, it is equally feasible that with dedicated effort then even islands with high reinvasion rates (i.e. greater than one rat per annum) can be kept rat free (Russell et al. 2008). Handa fulfils these criteria and more importantly is an SPA, with large nationally and internationally important colonies of breeding seabirds, and therefore leaving the rats would be unacceptable. Currently there is a significant risk of exponential increase of rats should the rats be left without further intervention.
- If greater resource could be applied to surveillance monitoring and in particular incursion response, it is feasible that Handa could be maintained as rat free in the long-term following further dedicated rat removal efforts, as it is clear the primary cause for the failure of biosecurity measure previously was lack of resource to deal with incursions. The Applicant would commit the resource required to completely eradicate rats from Handa and maintain the site as rat-free throughout the operational lifetime of the proposed development, providing the resource for additional eradication efforts as required.
- As a fundamental principle, it is necessary to understand where the rats are coming from so that they can be controlled. Although the first records of rats re-occurring on Handa in 2012 were near the stepping-stone islets, it is unclear whether the rats are coming from Tarbet or elsewhere along the mainland. Identifying and controlling the source of the rats and establishing a rat free buffer area along the mainland would reduce future incursion risk.
- The relevant stakeholders are supportive of rat eradication, although continuing engagement, and engagement with a wider range of interested parties (e.g. the local fish farm) will also be undertaken to ensure support for the work is sustained over time.
- It is anticipated that the eradication phase will be conducted during the winter period by an eradication specialist in collaboration with SWT, as detailed within the Implementation and Monitoring Plan.
- The rodent eradication will be funded by the Applicant. The Applicant will also supply resource to maintain biosecurity at Handa throughout the operational lifetime of the Proposed Development. The resource would cover monitoring of bait stations, and any trapping required in future should incursions occur.
2.4.4. Conservation targets
- The benefits of rat eradication are likely to be influenced by a range of factors. These are listed in section 2.3.5. However, comparison of seabird numbers, distribution and productivity data from Handa gathered in years with and without rats provides some indication of the benefit that may be achieved by a second rat eradication programme.
- Since the rats have returned to Handa, the Puffins are again restricted to breeding on Great Stack, a huge 120 m tower of Torridonian standstone, which matches the height of the adjacent cliffs and which also supports a similar number of nesting ledges. The stack is almost impossible to climb, and it is believed that it has always remained free of rats. This would seem to be a reasonable assumption.
- There are other smaller stacks including Needle Stack and a couple of ‘stacans’, or little stacks, which may also be free of rats. However, all the key species breed on Great Stack and at other locations on the main island. Therefore, comparison of productivity data (when available) from Great Stack with that obtained from mainland breeding locations enables some assessment of the level of impact that rats may be having. However, it is acknowledged that there are other factors that could also account for the difference between Great Stack and the main island such as habitat quality and human disturbance. The similarity in habitat between the cliffs adjacent to Great Stack and Great Stack itself is notable, as both are structurally identical, formed from the same Torridonian sandstone and both with the natural ledge formations that form ideal nesting habitat for many seabird species. However, other cliffs and breeding locations around Handa are not necessarily comparable in terms of habitat. It is also not possible to separate the impact of human disturbance: Great Stack is completely inaccessible, whereas other breeding locations are at varying distances from the path.
- In all instances the predicted benefits are viewed as precautionary in the sense that they do not incorporate how additional birds supplied annually will contribute to the growth of the colonies over the project lifespan.
Puffin
- Puffin numbers increased from 472 Puffins in 1996 to 735 Puffins in 1997 (Figure 2.10). This equates to a 56% increase and a gain of ~44 Puffins per year. However, this rough measure of benefit does not adequately describe the effect that an increased number of adults, together with increased productivity would have on promoting colony growth. However, understanding this process fully would be complex as other factors such as the allee effect and density dependence would need to be incorporated. Equally it is also acknowledged that the initial 1997 rat eradication took place when Puffin numbers were increasing nationally, although the step increase following rat eradication is obvious (see Figure 2.10). It is also important to appreciate that Puffin started to colonise the main island immediately after rat eradication, strongly indicating that rats were restricting Puffin to Great Stack.
- It is considered realistic that this level of increase could be achieved again, along with recolonisation of the main island, especially if areas are protected from human disturbance in advance of the eradication. It is certainly feasible that the colony could, in time, return to the numbers observed in 2004 (735 individuals). Indeed, if conditions were favourable, Puffin could exceed these numbers. An NVC survey carried out in 1997 suggested that there is ~20 ha of maritime cliff habitat (excluding ledges) that would be available to Puffin. Assuming a burrow density of 0.5 burrows per m2, based on what has been observed at St Kilda (Harris & Rothery 1988) it is feasible in terms of habitat alone that Handa could support >100,000 Puffin burrows. Although there are many reasons why this may not be achieved in practice, habitat availability is clearly not a limiting factor with regards to achieving conservation targets.
- The benefits of rodent eradication for Puffin as achieved at other islands are discussed fully in Section 2.3.5. At present Puffins on Handa are restricted to Great Stack, which is a breeding location also favoured by other species, presumably because of the lack of rats (and possibly human disturbance). It is possible that productivity could improve if other breeding sites were made available through the eradication of rats from the main island. If nothing is done to improve conditions for Puffin and the current pattern of decline continues, which over the past 20 years approximately averages around 25% per year, then it is considered likely that Puffin will be lost from Handa as a breeding species by around 2030.
Guillemot
- Guillemot numbers did not increase following rat eradication in 1997 (Figure 2.7). Although it is likely that there will be some benefit to Guillemots from rat eradication, it is not expected that the effects would be as marked as for Puffin and Razorbill. Guillemots nest on cliff faces, laying a single egg on a narrow ledge rather than building a nest. In some locations these ledges may be accessible to rats, as rats will climb cliff faces, but in many instances they may not be.
- Guillemots breed in roughly 11 different locations around Handa, and their distribution has not changed significantly since rat eradication in 1997 until recent years. Figure 2.19 shows an increase in the number of Guillemots nesting on Great Stack from 2018 onwards, which is coincident with the sudden increase in rat numbers. Guillemots appear to have relocated from G4 (Am Bonair) to Great Stack, which is located nearby offshore. It is of note that Am Bonair is one of the locations that Puffins recolonised following rat eradication in 1997 (see Section 2.4.2), suggesting that rats may be able to access nests in this location. Maps of rat activity (see Figure A9 in Appendix) show that rat detections increased in this location in 2017 perhaps resulting in a lower number of nests in this area in 2018. In summary, it appears that at least some Guillemots may be impacted by rats and are being displaced from Am Bonair on the mainland to Great Stack. Numbers of Guillemot nests on Great Stack have increased from 361 AON in 2018 to 436 AON in 2021 (an increase of 21%).
- Productivity monitoring for Guillemot has been carried out since 1997 with monitoring plots located at Poll Ghlup Geodh, a sheer sinkhole formed from a collapsed sea cave behind the cliff edge, and on the Great Stack. Therefore, it is possible to compare productivity on the mainland against productivity on Great Stack after the first rat eradication, and more recently now rats have returned. However, there are caveats: Poll Ghlup Geodh (G9 on Figure 2.19) may well be difficult for rats to access due to the sheer nature of the cliffs. It is also impossible to control for the influence of human disturbance. Poll Ghlup Geodh lies on the footpath, although people cannot closely approach the birds.
- During the rat free years, Poll Ghlup appeared on most occasions to be the most successful breeding location in terms of productivity (Table 2.8). However, during the recent rat invasion productivity has generally been higher on Great Stack. It is of note that this change occurred between 2016 and 2017, which ties in with the step increase in rat activity shown in Table 3.10. The difference in productivity between Poll Ghlup and Great Stack without rats was 0.07, but with rats productivity at Great Stack was 0.01 higher. Assuming that productivity at Great Stack and Poll Ghlup are roughly equal when rats are present, then it could be estimated that rats decrease productivity by ~0.05. As mentioned previously there may be other factors that could also affect productivity, which it is not possible to separate further.
Figure 2.19: Number of Guillemots nesting in different areas around Handa Island from 2015-2021, during the period of rat invasion.
- The impacts of rats on Guillemot productivity may differ at other nesting locations, such as Am Bonair, based on their accessibility to rats. This is an area which would require further study to progress understanding about the impacts of rats on Guillemots across all the island nesting locations.
- However, even a small difference in productivity can result in an increase in numbers if applied to a colony the size of Handa. For example, based on the latest SWT count of 68,524 Guillemots, then even an increase in productivity of 0.05 would constitute an additional 1,713 additional fledglings per year. Using the mean age specific survival rates from Horswill & Robinson (2015), and assuming a mean age of breeding of 6 years (Harris et al. 2016) then this increase in fledglings would translate to the supply of 577 additional adults per year.
- Since historically Guillemot numbers have been much higher, numbering 98,686 individuals in 1990, then it is clear that Handa does support enough suitable habitat to accommodate the potential increases that rat eradication and implementation of biosecurity measures could deliver over the operational lifetime of the Proposed Development.
Source: Compiled from wardens reports SWT 1997-2002 inclusive.
Razorbill
- Razorbill numbers on Handa have declined significantly, although there are some indications of recovery (see section 2.4.1). Although specific monitoring plots are counted annually (in the same locations as the Guillemot monitoring counts), all island counts are only conducted every 5 years.
- Comparison of all island Razorbill counts between 1997 (the year of rat eradication) and 2001 show that Razorbills increased from 15,573 to 17,042, an increase of 1,469 individuals (or 9%). Although this is in line with national trends (see Section 2.4.2) there were notable increases in use of some specific areas. These included An Carn Dubh boulders where numbers increased from 394 to 704. It seems likely that the presence of rats was decreasing habitat quality. By 2019, numbers of birds in this area had declined to only 195 Razorbills. Habitats such as boulder fields have been recolonised in other islands where rats have been removed such as Ailsa Craig.
- Numbers of Razorbills nesting on the main island cliffs opposite the Great Stack also increased from 609 individuals in 1997 to 1,127 in 2001 (an 85% increase). It is of note that Puffins also recolonised these cliffs during this period, indicating that rats may be able to access at least some of the nests on these cliff faces. In 2019 there were only 555 birds in this area, in line with numbers from 1997.
- Similarly, numbers of Razorbills increased at Geodh Great Stack from 499 birds in 1997 to 757 birds in 2001 (an increase of 52%). In 2019 this count area was combined with counts from Great Stack where numbers had significantly declined (see above).
- Numbers of Razorbills at Goedh Dearg increased from 740 in 1997 to 1182 in 2001 (an increase of 60%) suggesting that this usage of this area increased following rat eradication. Recently numbers have significantly declined in this nesting location to just 490 nests in 2019 (a decline of 141%), again suggesting that rats may be able to access this area.
- Although the Razorbill population on Handa did not increase following rat eradication, it seems likely that rats were preventing Razorbills from using some of the available (and otherwise suitable) habitat on the main island including An Carn Dubh boulders, the cliffs opposite Great Stack and Geodh Great Stack. It is likely that use of these areas would increase should rats be removed. However, annual all island counts and productivity monitoring of different areas of representative habitat would be required to quantify the benefits of rat eradication.
- Since Razorbills nest either on lower cliff ledges or among boulders at the bottom of cliffs, then significant improvements to breeding success following rodent eradication are anticipated. Indeed, there is evidence of increases in Razorbill numbers at 5 islands where they have been monitored following rodent eradiation, namely Canna, Lundy, Ramsey, the Shiants and Ailsa Craig (Thomas et al. 2017a, Brooker et al. 2018, RSPB News from the Rock 2021). However, Razorbill has been increasing nationally and so caution is required in interpreting this information.
- For example, rodents were removed from the Shiants in 2015, and by 2018 productivity for Razorbill increased from 0.72 to 0.79. Assuming a similar rise in productivity of 0.07 for Handa and based on the latest SWT count of 5047 individuals, then an increase in productivity of 0.07 would result in an additional 353 fledged chicks per year. Using the mean age specific survival rates from Horswill & Robinson (2015), and assuming a mean age of breeding of 4 years (Lavers et al. 2008) then this increase in fledglings would translate to the supply of 160 additional adults per year.
- Since historically Razorbill numbers have been much higher, numbering 16,394 individuals in 1990, then it is clear that Handa does support enough suitable habitat to accommodate the potential increases that rat eradication and implementation of biosecurity measures could deliver over the operational lifetime of the Proposed Development.
Kittiwake
- Kittiwake productivity monitoring is carried out at eleven plots annually. All island Kittiwake counts are carried out every five years. The timing of the counts (1999 and in 2005) is such that the impacts of sandeel failure from 2004 onwards dominate the dataset, which broadly reflects national trends (see Figure 3.3). However, it is possible to compare productivity between the monitoring plots on the main island and on Great Stack both from 1997-2002 representing the period after rat eradication and from 2015-2021 when rats had returned. This was done using a Wilcoxon signed rank test which showed that birds nesting on Great Stack were significantly more successful than those nesting on the main island both when rats were present on Handa (2015-2021)[17] and when they were absent (1997-2002)[18]. This suggests that the Great Stack represents superior habitat for Kittiwake over both time periods (Table 2.9).
Table 2.8. Productivity (number of chicks fledged per nest) of Kittiwake nesting on Great Stack and the main island during periods when rats were absent (1997-2001) and present (2015-2019)
Source: Compiled from wardens reports SWT 1997-2002 inclusive.
- However, the size of the difference is greater during the period between 2015-2021 (average difference =0.30) compared with 1997-2002 (average difference =0.19). This suggests there is an increase in average difference in productivity between the main island and Great Stack of 0.11. It is suggested that this could be attributed to rats, although may also be influenced by other factors (e.g. human disturbance) which cannot be separated out.
- Based on the last all island Kittiwake count numbering 3,749 AON from 2018, and assuming that 31% of Kittiwakes nest on Great Stack and 61% on the mainland (as in 2021[19]) then the 2,287 pairs nesting on the mainland could increase their productivity by 0.11, resulting in an additional 251 birds fledging per year. However, this data is based on comparison of two sites, and comparison of productivity between other nesting sites around Handa Island is required to generate a fuller understanding of the impacts of rats on Kittiwakes. Using the mean age specific survival rates from Horswill & Robinson (2015), and assuming a mean age of breeding of 4 years (Coulson 2011) then this increase in fledglings would translate to the supply of 124 additional adults per year.
- Since historically Kittiwake numbers have been much higher, numbering 125,000 individuals in 1990, Handa clearly does support enough suitable habitat to accommodate the potential increases that rat eradication and implementation and biosecurity measures could deliver.
Summary
- The benefits that may be delivered by rat eradication at Handa for the key species are summarised in Table 2.10. However, these numbers are potentially an underestimate of the benefit that may actually be delivered. As more adults are added to the population, the number of additional fledged chicks (and therefore adults) increases over time. The impact of the regular addition of these adults and their contribution to colony productivity is not recognized by these calculations. It is also considered likely that once rat free the numbers of adults that choose to nest on Handa would increase due to redistribution of birds from other local colonies (as appeared to occur after rat eradication in 1997 – see Figure 2.10). The contribution of these birds to the growth of the Handa colony is also not captured by this approach.
- Conservation targets for Handa have been set by multiplying the number of additional adult birds per year that would be generated by rat removal (as calculated above) by the 35 year project lifespan, and then adding to this the number of birds that are currently present. In all species except Puffin, historic maximum counts exceed conservation targets, demonstrating that Handa has the habitat to support these increases. The only exception to this is Puffin. However, as mentioned above Handa has the habitat to support >100,000 Puffin burrows and therefore habitat availability is not considered to be a limiting factor. Although the conservation target for Puffin exceeds historic maximum counts, it is considered to be realistic in view of the success of other rat eradication projects for this species (see Section 2.3.5).
2.4.5. Benefits to other species
- It is considered feasible that the eradication of rats will facilitate the return of Arctic Tern to Handa as a regularly breeding species once again. It is also possible that Common Tern could breed again too. Arctic Tern numbers increased significantly following rat eradication in 1997 (see Figure 2.17). The recolonisation of Feno Island (Terceira, Azores) by Common Terns and Roseate Terns was achieved following rat eradication (Amaral et al. 2010). Since terns are ground-nesting then the impacts of rats will be significant and improvement following eradication would be anticipated based on previous experiences from both Handa and elsewhere.
- It is unclear to what extent rat eradication will improve the breeding success of Fulmar, although some benefit would be anticipated. Rat eradication has resulted in improved breeding success of Fulmar at Lundy (Brooker et al. 2018) and Ailsa Craig[20], although not at Canna where the nesting area is not considered to be very accessible to rats, and where Fulmar numbers have continued to decline despite improvements in many other species (Luxmoore et al. 2019).
- At Handa there was some evidence of increased chicks fledging from the cliff top nest sites in 2000, although (like many of the initial improvements observed on Handa) this was not sustained. On Handa Fulmars nest in four locations: Great Stack, Little Stack, Puffin Bay and Fulmar Bay. Following rat eradication in 1997 the distribution of nests shifted, and Fulmar numbers increased in Fulmar Bay. Fulmar Bay is now the smallest nest site with 10 nests in 2021 compared to 41 on Little Stack, 52 on Great Stack and 49 in Puffin Bay. Comparison of productivity between these sites suggests that Fulmar Bay may well be impacted by rats (see Figure 2.20), with productivity reduced by about 0.20 relative to the other sites.
Figure 2.20: Mean productivity of Fulmars nesting in different locations on Handa Island. Data from 2015-2021 (excluding 2020 when data could not be gathered).
- It is not anticipated that Puffin Bay is impacted by rats, and this perception is reinforced by historic data, which shows a continuation of the trend of decline with no improvement following rat eradication in 1997 (Figure 3.21).
Figure 2.21: Fulmar AOS counts at Puffin Bay, Handa Island (1983-2005).
- Although this is not a species that has been studied in relation to rat eradication from islands, it is anticipated that there would be a benefit to Red-throated Diver. There are usually several pairs that breed on Handa (5 pairs in 2021 – SWT 2021).
- Eradication of rats has been of benefit to Shag on Ailsa Craig where they colonised following rat eradication and Canna, where numbers increased particularly in boulder field habitats (Luxmoore & Bell 2019). On Lundy, where Shag had been in decline, the eradication of rats served to halt the decline resulting in a stabilisation of numbers (Brooker et al. 2018). There is some evidence of this type of pattern on Handa following rat eradication in 1997 (Figure 3.13) before numbers declined sharply after 2001. On this basis it is anticipated that there would be a benefit to Shag from removing rats, as numbers are now very low. During the all island Shag count conducted in 2020 there were only 16 AON left, which represents a 50% decline since 2015. If rat eradication could halt this decline, it could prevent this species from being lost from Handa.
- Common Gull benefitted previously from rat eradication in 1997 and it is considered likely increased numbers could again be achieved. Numbers are currently very low (9 pairs), and like Shag, could be lost from Handa as a breeding species if the trend of decline continues. Some benefit to Herring Gull and Great Black-backed Gull is also considered possible, although numbers of both on Handa are relatively low.
- It is unclear whether rats are impacting on either Great or Arctic Skua, and this is an area that requires further study.
- It is anticipated that rat eradication would bring obvious benefit to ground nesting species present on Handa such as Oystercatcher and Ringed Plover. Benefits to these species were recorded after rat eradication in 1997. Dunlin and Redshank were also observed breeding for the first time on Handa after 1997, although now they no longer do. It is possible that these and/or other shorebirds could colonise and breed if Handa were free of rats again. Benefits post 1997 were also recorded for Eider and Shelduck.
- Other breeding species that occur on Handa considered likely to benefit from rat eradication include (but are not limited to) Cormorant, Grey Heron, Red Grouse, Snipe, Rock Dove, Skylark, Meadow Pipit, Pied Wagtail, Dunnock, Wren, Blackbirds, Song Thrush, Robin, Stonechat, Wheatear, Goldfinch and Willow Warbler.
- Since rat eradication did not previously result in the recolonisation of Storm Petrel, Black Guillemot or Manx Shearwater as had been speculated, then it is not anticipated that this would occur. However, it is difficult to predict what may or may not occur and the continued sighting of Black Guillemot does offer some hope.
- Following rat eradication in 1997 there were increased sightings of Pygmy Shrew. Since rats are a predator, then increased Pygmy Shew numbers would be anticipated. This is a phenomenon that has also occurred on Ailsa Craig and Lundy (Thomas et al. 2017a).
- It is also considered likely that rat eradication would benefit invertebrate populations on Handa. There are several notable butterfly and moth species, and it is considered possible that reduced predation of caterpillars by rats could be beneficial in increasing abundance and species richness of lepidoptera.
- It is also likely that Rabbits will increase following rat eradication as has happened on the Shiants, where some control of the increasing rabbit population has been required. After the 1997 rat eradication Rabbit numbers were low (possibly they were vulnerable to warfarin poisoning) but increased rapidly in the absence of rats. This then resulted in grazing of Sea Rocket beds, which had increased rapidly following rat eradication. The possibility of controlling rabbits should be considered at the project design stage to avoid similar scenarios reoccurring. It may not be necessary to control Rabbits if there are no apparent detrimental impacts, but it is necessary to be able respond rapidly to changing situations as appropriate.
2.4.6. Timescale & mechanism for delivery
- Full details of how the project will be delivered are included within the Monitoring and Implementation Plan, and only a brief summary is included here.
- It is anticipated that the initial rat eradication phase will be undertaken by an eradication specialist during the winter months, with surveillance and seabird monitoring conducted by a qualified contractor, who would also be responsible for implementing incursion response plans should an incursion occur.
- Since stakeholders are positive, it is not anticipated that there are any significant barriers to implementation, which could be undertaken relatively rapidly (although the eradication phase itself would need to be undertaken during the winter months).
2.4.7. Additionality & uncertainty
Additionality
- Since Handa Island is an SPA where biosecurity work is already underway then the issue of additionality does need to be addressed. This issue was raised by the RSPB following the final stakeholder consultation meeting on 22nd September[21]. They suggested that the removal of rats from Handa would not be an appropriate compensatory measure as commitments to biosecurity should already be incorporated within the current SPA management objectives. Whilst Handa does have management objectives related to maintaining biosecurity, these are to monitor for the presence of Brown Rat and control if necessary. They do not mention the eradication of Brown Rat, implying that their aim is to minimize rat numbers only.
- The recent invasion of rats in 2012 clearly demonstrates that the level of resource SWT are currently able to dedicate to biosecurity is not sufficient to control rat numbers. This is demonstrable through reference to the wardens reports (SWT 2016-2020), which show that staff were aware of the developing problems with rats, but were unable to dedicate staff time to setting and checking the large number of traps required to control what was clearly a rapidly increasing rat population.
- The lack of financial resource to charter the ferry to check bait stations deployed on one of the stepping-stone islets was also a problem (SWT 2012). Although the bait stations were installed in 2012 as it was understood that the islets were highly likely to be the route of recolonisation, there was no resource to carry out checks of these bait stations.
- Both EC and national guidance on compensatory measures indicates that measures that are accepted as ‘normal’ practice should not constitute compensation. ‘Normal’ practice is defined as being within the bounds of everyday financial and political realities. However, if normal practice is failing, and the measures suggested are additional (i.e. over and above what can reasonably be expected to happen in the absence of the project), then the work can be classified as appropriate compensation.
- Although SPAs would be expected to maintain biosecurity by writing a biosecurity plan to minimise the chance of incursion and to carry out surveillance monitoring, it is argued that maintaining biosecurity at an island such as Handa will always require resource over and above what may typically be required at other islands. This is due to a combination of factors: its proximity to the mainland, the presence of several ‘stepping-stone’ islets between Handa and the mainland, its large size, the number of visitors, and the availability of ample habitat and natural food resources for rodents. For these reasons, coupled with the high conservation value of the site for seabirds, significant additional effort will always be required to control rats at this site.
- The range of potential measures under consideration for Handa are by any definition over and above the current management objectives for the site, which at present offer no commitment to rat eradication in spite of the obvious need for rats to be removed both due the high conservation value of the seabird colonies and the known negative impacts of rats on seabird islands.
- SWT currently employ one paid warden on a seasonal basis to manage the reserve at Handa. The warden is assisted by five volunteers. The warden and volunteers run the reserve, carry out seabird counts and productivity monitoring, meet visitors and ensure they keep to the paths (several ferry trips a day), man a small souvenir shop, and carry out school visits, repair work and general maintenance of the bothy area.
- There is therefore currently little resource to dedicate to biosecurity. On occasions when rats have reoccurred the there is no resource to currently fund further systematic rodent control or eradication efforts. This has ultimately led to reinvasion of rats and a return to high levels of rat activity
- The Applicant would supply the relevant resource to deal with all eradication, monitoring and elements of biosecurity. This would include a resource to undertake rat eradication work if further incursions of rat and other invasive mammalian predators occur. Since rat chew stations are currently monitored, there is a small element of additionality with regards to this single element. However, at present surveillance visits are carried out monthly during the breeding period. A dedicated biosecurity resource would facilitate improved surveillance with increased frequently of visits including during the winter period. Other tasks such as looking for scat, carrying out lamping for hedgehogs, and trapping for rats and routine checking of any traps that are set could also be carried out routinely with additional resource.
- Funding would also be supplied to coordinate a dedicated rat eradication in the event of future invasion. This is of particular importance, since failure to fund incursion response can be viewed as the primary cause of failure previously. The funding would also facilitate the sustained stakeholder engagement required for the biosecurity work to be successful, enabling SWT to engage with a wider range of stakeholders than has been undertaken previously.
Uncertainty
- There is reasonable certainty that rats can be eradicated from Handa, as this has previously been achieved successfully. However, there is uncertainty around whether the current A24 traps are effective. However, alternative methods of rat eradication will be pursued.
- Future incursions and potential reinvasions are a risk, and indeed would be anticipated at a site such as Handa. However, the additional resource provided by the project would enable both continuous surveillance and rapid response to such events.
- There is some uncertainty regarding the conservation targets set in Section 2.4.5. Quantifying the benefit rat eradication is difficult; comparison of counts before and after rat eradication may be of limited use as it is impossible to separate out the influence of other factors, such as mass prey failures, which may have a significant impact on the dataset. This point applies to Puffin only as productivity data has been used to predict benefits for all other species.
- Applying data from one site to make predictions about another is problematic as the islands may be subject to different pressures and may therefore respond differently to rodent eradication. This point applies only to Razorbill, where data from the Shiants has been used to inform predictions for Handa.
- However, predictions for Kittiwake and Guillemot are probably as accurate as can reasonably be expected as they are based on comparisons with rat and rat free nesting areas and use long-term site-specific productivity datasets. However, in all instances the predicted benefits are viewed as precautionary in the sense that they do not incorporate how the additional birds supplied annually will contribute to the growth of the colonies over the project lifespan.